2807
Multi-echo sliding interleaved projection reconstruction (SLIPR) imaging of the carotid artery1Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Siemens Healthcare, Salt Lake City, UT, United States, 3Department of Veterans Affairs (VASLCHCS), Department of Veterans Affairs (VASLCHCS), UT, United States
Synopsis
An optimized 3D stack of stars with Sliding Interleaved Projection Reconstruction (SLIPR) provides improved carotid MRA over conventional Time of Flight (TOF) techniques. The stack of stars SLIPR technique takes better advantage of the inflow effect to maximize contrast to noise between blood and tissue. In addition, a multi-echo radial readout minimizes off resonance blurring, allows for fat water separation and shortens acquisition time compared with non-sliding 3D TOF techniques.
Purpose
Overlapped slab acquisition1 combines many advantages of multislice 2D and single slab 3D techniques but still suffers from slab boundary artifacts. Sliding slab techniques such as SLINKY2 and SLIPR (Sliding Interleaved Projection Reconstruction)3 yield greatly reduced slab boundary artifacts but have seen limited clinical adoption. However, with the desire for larger FOV imaging and increased spatial resolution, there is a renewed interest in sliding slab techniques4-6. This paper evaluates the relative performance of SLIPR and non-sliding 3D MRA techniques7 in application to carotid artery imaging.Methods
All studies were performed on a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, DE) after IRB approval and informed consent of all subjects. Data was acquired with a custom 7 element carotid receive coil with a 0.8mm x 0.8mm x 1mm resolution, 6/8 Partial Fourier in the partition direction. For the 3D Time of Flight (TOF), 3 slabs with 48 partitions, 20 degree TONE pulse, 20.83% overlap and 16.7% oversampling provide 12.4 cm of slice coverage. For SLIPR, 128 thin slabs with 8 partitions and 25% oversampling provide 13.2 cm of coverage. Total image time for the 3D TOF is 3m26s while the image time for SLIPR is 3m35s with 96 total rays per slice (with golden angle spacing). No fat saturation was applied in either sequence. Venous suppression was not yet available for the SLIPR sequence.Results and Discussion
Inflow MRA techniques saturate stationary tissue with repetitive RF-pulses while inflowing blood has a high initial magnetization. The excited volume thickness should be kept to a minimum to maximize the contrast between inflowing blood and saturated tissue signals. However, for 3D TOF, utilizing multiple thin slabs in the place of one thicker slab produces a well-known slab boundary artifact due to imperfect slab profiles and patient motion. The SLIPR technique3 overcomes this limitation by only exciting a thin slab that slides across a larger 3D volume (Figure 1). This work images 130 mm of coverage in the slice direction with a 1mm slice resolution while only ever exciting an 8mm slab.
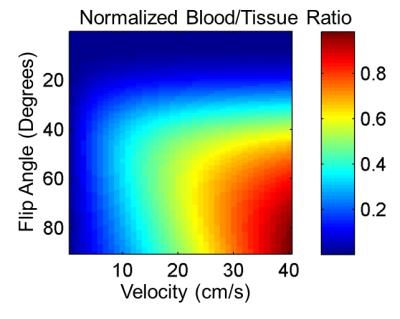
TR is selected to ensure maximum inflow of blood for this slab thickness. For carotid artery percent stenosis of about 50%, end diastolic velocities are about 40cm/s with peak systolic velocities over 100cm/s.8 For SLIPR, a TR of 20ms ensures complete inflow across the 8mm slab even at end diastole (Figure 2). A multiecho readout (Fig. 2d) is also selected to increase sampling bandwidth to minimize off resonance blurring and to allow for fat water separation using IDEAL9. The short T1 of fat makes it difficult to suppress using just the inflow technique. If blood in the SLIPR slab is completely refreshed each TR then the optimal flip angle would be a 90 degree excitation. However, a lower flip angle is used to accommodate slower velocities in healthier patients, smaller branch vessels and vessel orientations that are in plane rather than completely through plane. From experience, a flip angle of 40 degrees maintains good blood to tissue contrast for high velocities and maximizes the same contrast for slow velocities (Figure 3).
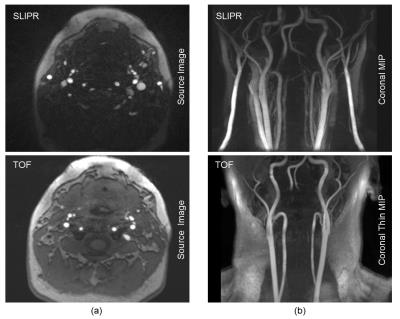
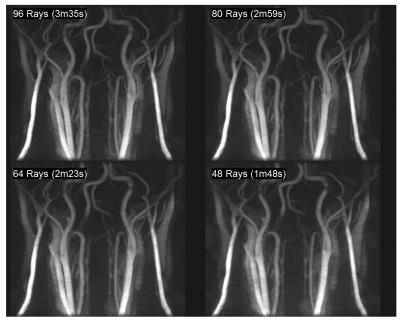
Figure 4 compares of a multi-slab 3D TOF sequence with an equivalent SLIPR technique. Source images are shown in Figure 4a with coronal MIPs shown in Figure 4b. A thin MIP is required for the TOF because the fat signal obscures the vessels when a standard MIP is used. While the thin MIP improves the blood to tissue contrast in SLIPR, it is not necessary for vessel visualization due to the IDEAL fat/water separation. To minimize boundary artifacts, the TOF protocol utilizes a larger slab thickness (4.8 cm) while the SLIPR protocol uses a much thinner 8 mm slab thickness. Consequently, the SLIPR technique has improved inflow between excitation pulses resulting in better contrast to noise between blood and tissue. Since MR angiograms are sparse in image space, it is possible to use even fewer rays than the 96 rays per slice used in this work (to match the acquisition time of the 3D TOF). As seen in Figure 5, even using 48 rays per slice (half the image time of the 3D TOF) yields acceptable results.
Conclusion
The results presented demonstrate the feasibility of using SLIPR for large FOV carotid artery MRA and show improved results over a 3D TOF protocol. With venous suppression (available soon for SLIPR) and the ability to achieve better blood to tissue contrast in a shorter acquisition time, the carotid artery SLIPR protocol is a clinically viable alternative to non-sliding 3D MRA techniques.Acknowledgements
This work was supported with resources from the George E. Wahlen Department of Veterans Affairs Medical Center (Salt Lake City, Utah) as well as funding from Ben B. and Iris M. Margolis Foundation, Cumming Foundation, Mark H. Huntsman Endowed Chair, Siemens Medical Solutions and NIH R01 HL127582 and RSNA Research Scholar Grant RSCH1414.References
1. Parker DL, Yuan C, Blatter DD. MR angiography by multiple thin slab 3D acquisition. Magn Reson Med 1991;17:434–451.
2. Liu K, Rutt BK. Sliding interleaved ky (SLINKY) acquisition: A novel 3-D MRA technique with suppressed slab boundary artifact. J Magn Reson Imag 1998;8:905–911.
3. Parker DL, Roberts JA, Alexander AL, Goodrich KC, Tsuruda J. Magnetic Resonance Angiography With Sliding Interleaved Projection Reconstruction (SLIPR) Acquisition. J Magn Reson Imaging 1999;10:569–575.
4. Kwon KT, Kerr AB, Wu HH, Hu BS, Brittain JH, Nishimura DG. Non-Contrast-Enhanced Peripheral Angiography Using a Sliding Interleaved Cylinder Acquisition. Magn Reson Med 74:727–738.
5. Choi J, Seo H, Lim Y, Han Y, Park H. Sliding Time of Flight: Sliding Time of Flight MR Angiography Using a Dynamic Image Reconstruction Method. Magn Reson Med 73:1177–1183.
6. Li Z, Wang D, Robison RK, Zwart NR, Schar M, Karis JP, Pipe JG. Sliding-Slab Three-Dimensional TSE Imaging With a Spiral-In/Out Readout. Magon Reson Med 2016;75:729.
7. Suryan G. A time-of-flight method. Proc Indian Acad Sci Sect 1959;A33:107.
8. Rodriguez G, Arnaldi D, Campus C, Mazzei D, Ferrara M, Picco A, Fama F, Colombo BM, Nobili F. Correlation between Doppler Velocities and Duplex Ultrasound Carotid Cross-sectional Percent Stenosis. Acad Radiol 2011;18(12):1485-91.
9. Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, Pelc NJ. Multicoil Dixon Chemical Species Separation With an Iterative Least-Squares Estimation Method. Magn Reson Med 51:35–45, 2004.
Figures