2801
Objective Measurement of Carotid Lumen Using Non-Contrast SNAP MRA1Department of Bioengineering, University of Washington, Seattle, WA, United States, 2Department of Radiology, University of Washington, Seattle, WA, United States, 3Department of Surgery, University of Washington
Synopsis
Maximum intensity projection (MIP) is a commonly used tool to measure luminal stenosis. MIP images may underestimate stenosis. Directly measuring lumen size represents an alternative solution, but manual segmentation is laborious and sensitive to flow artifacts and bias. Simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP) imaging affords a novel 3D non-contrast MRA approach. The polarity map available with SNAP makes it less sensitive to flow artifacts and facilitates objective lumen boundary definition. In this study, objective lumen measurements on SNAP and 3D-TOF are compared using CE-MRA as a reference. Results showed good agreement between SNAP and CE-MRA on lumen assessment.
Purpose
Maximum intensity projection (MIP) of MR images is a widely used technique to measure luminal stenosis. However, MIP image can underestimate luminal stenosis and is sensitive to threshold selection. Luminal stenosis can be better evaluated with measurement of lumen size on the original source images. Manual outlining of the lumen contour, however, is laborious and sensitive to artifacts and unaided subjective lumen outlines are more dependent on individual reviewer bias and window-level settings. Simultaneous non-contrast angiography and intraplaque hemorrhage MRI (SNAP) is a high-resolution 3D non-contrast MRA approach. By restoring polarity map, SNAP can simultaneously detect lumen (negative) and intraplaque hemorrhage (positive). This polarity map makes SNAP MRA less sensitive to flow artifacts1. Additionally, it allows the lumen boundary to be determined objectively and therefore reduce the bias of individual reviewers. In this study, the accuracy of carotid lumen area measurements with the objective lumen outlines on SNAP was compared with objective measurement on 3D TOF using contrast enhanced magnetic resonance angiography (CE-MRA) as the reference standard.Method
Patient recruitment: Eleven subjects (9 men, 2 women; mean age, 72.1 years ±8.6 [±standard deviation], age range, 58-85 years) were recruited for this study with Institutional Review Board approval and written informed consent. Subjects were enrolled if they had 16-79% stenosis by duplex ultrasonography in at least one carotid artery.
MRI Scan: Bilateral carotid arteries were scanned using a dedicated carotid coil for SNAP and 3D TOF and a body coil for CE-MRA. Scan parameters were: CE-MRA: TR/TE: 5.46/1.69ms, FOV: 350 (FH)×350(RL)×64(AP)mm3, resolution: 0.8×0.8×0.8mm3. SNAP: TR/TE: 9.8/4.72ms, TI: 500ms, FA: 11o, FOV: 160(FH)×160(RL)×32(AP)mm3, resolution: 0.8×0.8×0.8mm3. 3D TOF: TR/TE: 24/4.86ms, FA: 20o, FOV:160(AP)×160(RL)×46(FH)mm3, resolution: 0.6×0.6×2.0 mm3.
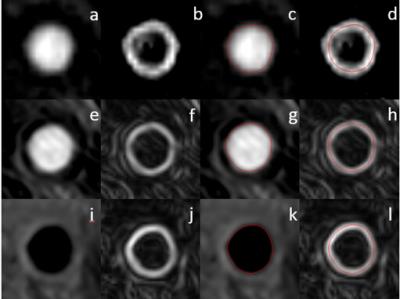
Image Review: Images were first reformatted into axial slices with thickness of 2mm. 21 slices (one at the carotid bifurcation, ten above, and ten below) of each artery were included in the review. Images of the three sequences were reviewed in independent sessions. The order of the reformatted slices was randomized to avoid recall bias and remove the influences from adjacent slices. A custom-designed image analysis software2 was used to outline lumen boundary and calculate lumen area. For each slice, a gradient map was generated with Sobel filter (Figure 1), which was used to determine lumen boundary on TOF and CE-MRA (maximum gradient). For SNAP, lumen boundary was determined by the boundary of negative signal region. The degree of stenosis at the artery level was also calculated, as the ratio of the minimum lumen area to the respective lumen area of the most proximal slice.
Statistical Analysis: Agreement was summarized using the intraclass correlation coefficient (ICC). Bias and agreement were also evaluated qualitatively using Bland-Altman plots. Analyses were performed using all slices as well as within subgroups categorized based on presence of atherosclerosis (diseased or healthy).
Results and Discussion
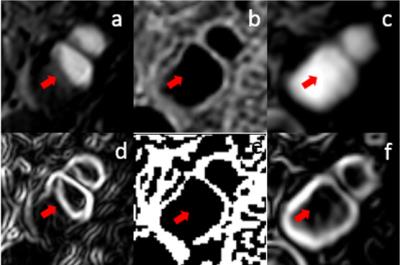
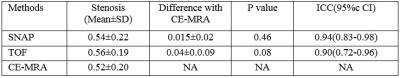
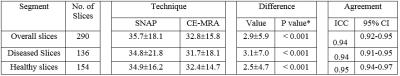
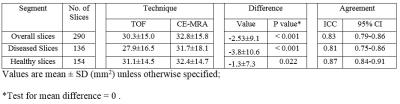
A total of 15 carotid arteries showed luminal stenosis and were analyzed. 25 slices out of the FOV of either TOF (17) or SNAP (8) images were discarded. As shown in Table 2 and 3, agreement in lumen size measurements by SNAP and CE-MRA was excellent (ICC: 0.94). Relative to CE-MRA on lumen measurement, there was a positive bias of SNAP (mean difference: 2.9 mm2) (Table 2) and a negative bias of TOF (mean difference: -2.5mm2). The bias of TOF appears smaller in the healthy group than that in the diseased group whereas SNAP showed similiar bias for both the two subgroups. The stenosis measurements on SNAP and TOF both demonstrated a good agreement with CE-MRA (Table 1). Nonetheless, SNAP appeared to be slightly higher than TOF using CE-MRA as reference (ICC=0.94, 95% CI: 0.83-0.98 vs ICC=0.90, 95% CI: 0.72-0.96). The reason for relative larger lumen size (Table 2) with SNAP measurements is that the maximum gradient is within the lumen which leads to smaller lumen size than its actual value while the polarity map of SNAP can directly define lumen/vessel wall boundary. The reason of the negative bias of TOF measurement (Table 3) is that contrast of TOF is sensitive to flow rate and flow artifacts3 compared with CE-MRA (Figure 3). These results indicate that SNAP provides an alternative non-contrast MRA method to 3D-TOF with possible advantages for objective lumen segmentation.Conclusion
SNAP showed excellent agreement with CE-MRA for both lumen area and stenosis with objective measurements. With polarity map, SNAP MRA was less sensitive to flow artifacts than 3D-TOF MRA. Moreover,these results show that non-contrast SNAP MRA has the potential for convenient automatic lumen area and stenosis measurements methods development in the future.Acknowledgements
References
1. Wang, Jinnan, et al. "Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation." Magnetic resonance in medicine 69.2 (2013): 337-345. 2. Kerwin, William, et al. "Magnetic resonance imaging of carotid atherosclerosis: plaque analysis." Topics in Magnetic Resonance Imaging18.5 (2007): 371-378. 3. Nederkoorn, Paul J., et al. "Time-of-flight MR angiography of carotid artery stenosis: does a flow void represent severe stenosis?." American journal of neuroradiology 23.10 (2002): 1779-1784.Figures