2799
Coronary MRI allows Assessment and Monitoring of Coronary Patency and Blood Flow Velocity Quantification in Patients treated with Bioresorbable Vascular Scaffolds1Department of Radiology, Medical Physics, Medical Center - University of Freiburg, Freiburg, Germany, 2Department of Cardiology and Angiology I, University Heart Center, Freiburg, Germany
Synopsis
Bioresorbable Vascular Scaffolds (BVS) provide a new and rapidly evolving alternative to drug eluting metal stents (DES) in the treatment of coronary artery disease. Besides potential advantages over DES in the restoration of the vessel function and reduction of post-interventional angina BVS allow for artifact-free coronary MRI of scaffolded arteries. In this study, we demonstrate that MRI for non-invasive monitoring of coronary arteries after BVS implantation is feasible by assessing coronary patency in a group of 11 patients initially and one year post-intervention.
Introduction
Coronary MRI is a non-invasive and radiation-free imaging technique that allows for the assessment of vascular patency and detection of stenoses in patients with coronary artery disease (CAD).1 After treatment of CAD patients with percutaneous coronary intervention (PCI), about 30% of these patients re-develop symptoms of angina pectoris within the first year post-intervention.2 Thus, coronary MRI would be desirable for the follow-up of vascular patency after PCI. However, imaging of arteries treated with metallic drug eluting stents (DES) is not feasible due to strong imaging artifacts.3
Bioresorbable Vascular Scaffolds (BVS) provide a new and rapidly evolving alternative to DES in the treatment of CAD. Besides potential advantages over DES in the restoration of the vessel function and reduction of post-interventional angina, BVS allow for artifact-free coronary MRI of scaffolded arteries. This has been shown in proof-of-concept studies at 1.5T and 3T4-6.In this work, we further assess coronary patency in a group of 11 patients one year after the treatment with BVS and demonstrate the applicability of coronary flow measurements in the presence of BVS for myocardial blood flow quantification.
Methods
Eleven patients were included that received one or more BVS in proximal segments of the left anterior descending (LAD), left circumflex (LCX) or right coronary artery (RCA). The baseline MR exam was performed within three days post-intervention and all patients were imaged again after 12 months with the same MR protocol.
All MR image data were acquired using ECG triggering, and 3D acquisitions were additionally gated to end-expiration. Coronary arteries were localized using a whole-heart low-resolution 3D navigator-gated bSSFP sequence with fat-saturation. This was followed by a targeted 3D bright-blood navigator-gated FLASH sequence with 1.08 mm isotropic resolution, fat saturation and T2 preparation (TE/TR: 1.9/3.1 ms, FoV: 28x277x295x28 mm³, TET2prep: 40 ms, R = 2).
In a subgroup of 5 patients the ejection fraction was measured with a multi-slice cine-bSSFP sequence in short-axis view. To demonstrate proof-of-concept of coronary flow measurements in the proximity of the scaffold, time-resolved blood flow velocity quantification via phase-contrast MRI was performed in one patient both at baseline and after 12 months. Therefore, a cross-sectional 2D single slice positioned proximal to the scaffolded vessel area was acquired with through-plane velocity-encoding using spiral sampling, prospective ECG-triggering and respiratory navigator gating as well as a 1-2-1 binomial water selective RF excitation pulse (TE/TR: 4.8/15.2 ms, 16 interleaves, SL: 8 mm, FoV: 200x200 mm³, VENC: 35 cm/s).7
To evaluate lumen diameters and assess potential re-stenoses in the scaffolded segment of the artery, all bright-blood image sets were manually reformatted along the course of the vessel (IMPAX EE Extended Multi-planar Reconstruction, AGFA Healthcare). Based on these reformats, the lumen diameter was measured as the FWHM and averaged over the scaffolded segment.
Results
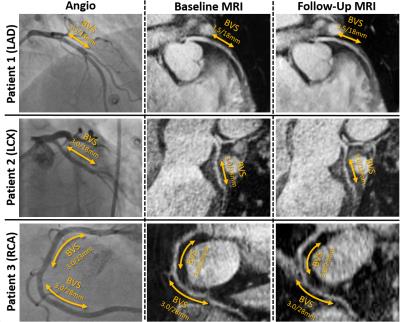
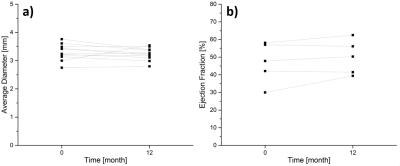
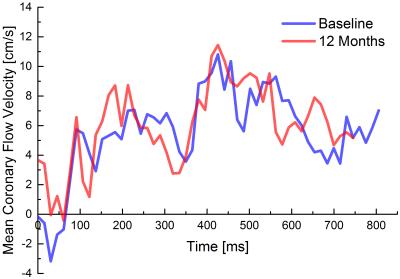
Imaging was successful in all patients in both the initial and follow-up exam. None of the patients suffered from adverse cardiac events within the first year post-intervention. Figure 1 exemplary shows imaging results of three patients: bright-blood images corresponded well to the angiography images showing prominent and open luminal areas both at baseline and after 12 months. No visually apparent occlusion is seen in any of the patients after 12 months. This is confirmed by the measured vessel diameters which show now significant difference between the initial and the follow-up measurement (cf. Fig 2a). EF results measured in 5 patients (Fig 2b) show a trend towards higher values, although not significant. The blood flow velocities measured in one patient are shown in Fig. 3. The flow pattern after 12 months (total flow: 65.5ml/min) is unchanged compared to baseline (69.7/ml/min). The flow velocity curves agree with previously published coronary flow MRI8 indicating that myocardial blood filling occurs mainly during diastole.Conclusion
With coronary MRI, luminal patency of scaffolded coronary arteries can be assessed and monitored in patients with bioresorbable vascular scaffolds. Additional functional insight may be provided by time-resolved blood flow velocity quantification via phase-contrast MRI although this remains to be studied in a larger group of patients. Also, further studies could aim for the application of improved coronary MRI such as compressed sensing techniques9 to include patients with BVS in distal vessel segments and measuring endothelial function with MRI10 as a biomarker for vascular constitution.Acknowledgements
Grant support by the Deutsche Forschungsgemeinschaft (DFG) under grant number BO 3025/2-2 is gratefully acknowledged.References
1. Kim WY, Danias PG, Stuber M, et al. Coronary Magnetic Resonance Angiography for the Detection of Coronary Stenoses. N. Engl. J. Med. 2001;345(26):1863-1869
2. Klemm T, Duda S, Machann J, et al. MR imaging in the presence of vascular stents: A systematic assessment of artifacts for various stent orientations, sequence types, and field strengths. J. Magn. Reson. Imaging 2000;12(4):606-615
3. Ben-Yehuda O Kazi DS, Bonafede M, et al. Angina and associated
healthcare costs following percutaneous coronary intervention: A real-world
analysis from a multi-payer database. Cathet. Cardiovasc. Intervent. 2016; doi:10.1002/ccd.26365
4. Baron-Rochette G, Vautrin E, Rodière M, et al. First magnetic resonance coronary artery imaging of bioresorbable vascular scaffold in-patient. Eur. Heart J. Cardiovasc. Imaging. 2015;16(2):229
5. Reiss S, Krafft AJ, Zehender M, et al. Magnetic Resonance Imaging of Bioresorbable Vascular Scaffolds Potential Approach for Noninvasive Evaluation of Coronary Patency. Circ Cardiovasc Interv. 2015;8(4):e002388
6. Reiss S, Krafft AJ, Menza M, et al. 3T Coronary MRI in Patients Treated with Bioresorbable Vascular Scaffolds for the Assessment of Vascular Patency and Blood Flow Velocity Quantification. Proc. Intl. Soc. Mag. Reson. Med. 2016
7. Menza M, Föll D, Hennig J, et al. Spiral SPIRIT Tissue Phase Mapping enables the acquisition of myocardial motion with high temporal and spatial resolution during breath-hold. Proc. Intl. Soc. Mag. Reson. Med. 2016
8. Keegan J, Raphael CE, Parker K, et al. Validation of high temporal resolution spiral phase velocity mapping of temporal patterns of left and right coronary artery blood flow against Doppler guidewire. J Cardiovasc Magn Reson 2015;17(1):1-13
9. Ginami G, Coppo S, Feng L, et al. Cardiac and Respiratory Motion-Resolved Free-Running Whole-Heart Coronary MRA of Patients Using 5D XD-GRASP Reconstruction. Proc. Intl. Soc. Mag. Reson. Med. 2016
10. Yerly J, Ginami G, Nordio G, et al. Coronary endothelial function
assessment using self-gated cardiac cine MRI and k-t sparse SENSE. Magn. Reson.
Med. 2015;doi: 10.1002/mrm.26050
Figures