2789
Comparison of Two Different Implementations for the Simultaneous Non-Contrast Angiography and Intraplaque Hemorrhage (SNAP) Sequence1Radiology and Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 2Seaman Family MR Research Centre, Foothills Medical Centre, Calgary, AB, Canada, 3Radiology, University of Washington, Seattle, WA, United States
Synopsis
We compare, using Bloch equation simulation, the relative signal in intraplaque hemorrhage (IPH), arterial wall (Wall) and Lumen (i.e., blood), along with the IPH–Wall contrast for two different implementations of the SNAP sequence. The first implementation is the original version (Ver1) that uses a linear phase-encoding scheme post-IR, whereas the second version (Ver2) uses centric slice-encodes post-IR. We show that, although Ver2 appears to be less time-efficient, it actually provides increased signal and contrast for the same acquisition time.
Purpose
Intraplaque hemorrhage (IPH) plays a critical role in the evolution of carotid atherosclerotic disease, and is strongly associated with increased risks of clinical events, especially neurological ischemic events. Moreover, luminal stenosis remains the current clinical standard for evaluating stroke risk due to carotid disease.
Recently, Wang and colleagues [1–3] developed a simultaneous non-contrast angiography and IPH technique (called SNAP) that allows both MR angiography (MRA) and IPH evaluation in the same acquisition. Briefly stated, SNAP is a 3D phase-sensitive IR-prepared spoiled gradient-echo acquisition that interleaves the volume (VOL) and the reference (REF, necessary for accurate phase-sensitive reconstruction) to generate images with negative signal corresponding to MRA and positive signal corresponding to IPH.
Wang’s implementation (Ver1) uses a time-efficient linear phase-encoding scheme post-IR. Here we compare a second implementation (Ver2) that instead uses a centric slice-encoding scheme post-IR. Albeit less time-efficient, we show that it actually provides equal or superior signal and contrast in the same 5-minute scan, with potentially less image blurring.
Methods
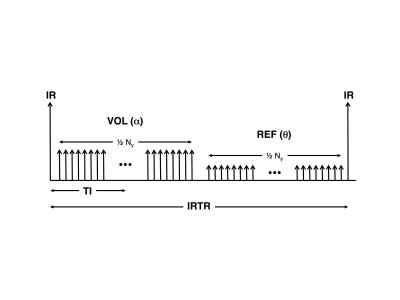
The Wang implementation (Ver1) is shown in Figure 1. The IR-to-IR time is defined as IRTR. Note that the REF acquisition has no preceding IR pulse, it uses a smaller flip angle, and it is acquired immediately after the VOL acquisition, making it very time-efficient. Also, note that the inversion time (TI) occurs midway during the alpha-train.
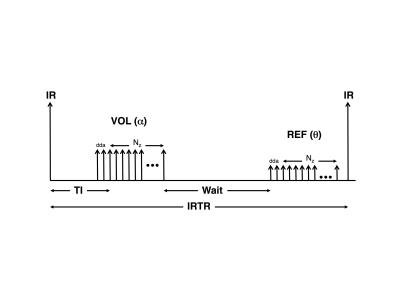
Conversely, the alternate implementation (Ver2), as shown in Figure 2, has some dead-time between the IR-pulse and the first alpha-pulse, the TI occurs immediately after the dummy data acquisitions (dda), there are only dda + Nz alpha-pulses (instead of ½ Ny), and there is significant dead-time between the VOL and REF acquisitions to make up IRTR. Unlike Ver1, which uses linear phase-encoding, Ver2 uses centric slice-encoding for VOL and reverse-centric slice-encoding for REF.
We performed a Bloch equation simulation for the two SNAP implementations. The tissue parameters were {IPH, Wall, Lumen} with T1 = {500, 1115, 1550} ms, T2* = {15, 20, 275} ms, and M0 = 1 for all tissues. The scanning parameters that were identical for both implementations included TE/TR = 2/10 ms, 192 x 192 x 40 acquisition matrix. Ver1 used IRTR = 1970 ms, TI = 500 ms, α/θ = 11º/5º, and 2 signal averages for a total scan time of 5:17. Ver2 had IRTR = 1650 ms, TI = 650 ms, α/θ = 12º/6º, and 1 signal average for a total scan time of 5:17. We report the relative Mxy values for VOL at time TI, and for REF when we are at the centre of k-space.
Results
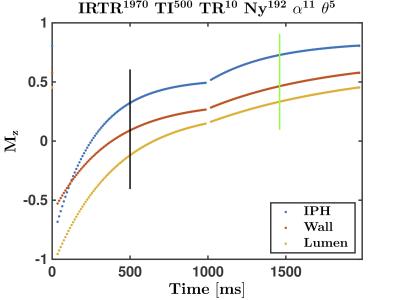
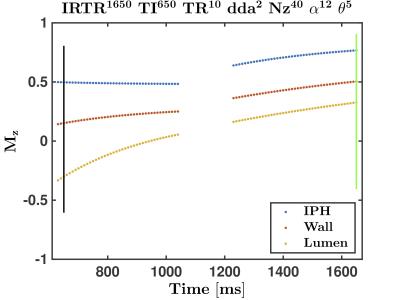
The Mz evolutions of Ver1 and Ver2 are shown in Figures 3 and 4, respectively. Note that the black and green vertical lines indicate, respectively, the TI for VOL and the centre of k-space for REF. In both implementations, the transverse magnetizations of the IPH and Wall at TI are positive, but negative for Lumen (i.e., blood), as required. Also, note the significant signal modulation for Ver1 as compared to Ver2. This should, in theory, provide less image blurring effects in Ver2 as compared to Ver1.
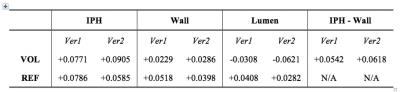
The relative signals and contrasts are shown in Table 1. Note that although Ver2 is seemingly less time-efficient owing to all the dead times (compare Figures 1 and 2), the signals in the IPH, Wall and Lumen are actually greater (in magnitude) than those of Ver1, and the IPH–Wall contrast is also higher. One drawback with Ver2 is that the REF signals in all tissues are about 70–75% compared to those from Ver1. This might compromise the phase-sensitive reconstruction accuracy slightly.
Conclusions
We showed that although the alternate implementation of SNAP using centric (for VOL) and reverse-centric (for REF) slice-encoding is less time efficient than the original linear phase-encoding scheme of SNAP, the expected signal and contrast for VOL are actually higher for the same scan time, and that it potentially provides higher quality images with less blurring.Acknowledgements
The support of the Canadian Institutes for Health Research and the Hopewell Professorship in Brain Imaging are acknowledged.References
[1] J Wang, M Ferguson, N Balu, et al. Improved carotid intraplaque hemorrhage imaging using a slab-selective phase-sensitive inversion-recovery (SPI) sequence. Magn Reson Med 2010, 64:1332–1340.
[2] J Wang, P Bornert, H Zhao, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med 2013, 69:337–345.
[3] J Wang, M Guan, K Yama, et al. In vivo validation of simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP) magnetic resonance angiography: an intracranial artery study. PLOS ONE, DOI:10.1371/journal.pone.0149130, February 10, 2016.
Figures