2784
3D Golden-Angle Spiral Sparse Parallel-Imaging for Lumen Area Measurements of the Entire Proximal and Mid-Segments of the Coronary Arteries in a Breath-Hold1Russel H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Coronary artery endothelial function (CEF) could historically only be measured with invasive catheterization-based testing. Recently, 2-dimensional cine MRI in combination with isometric handgrip exercise was introduced to quantify CEF noninvasively. However, the MRI technique only assesses CEF at one or two locations in an artery and does not allow assessment of regional heterogeneity present throughout diseased vessels. The aim of this work was to develop high-resolution isotropic 3-dimensional cine coronary MRI that enables measures of coronary lumen area along the entire proximal and mid segments of the coronary arteries in a single breath-hold, a pre-requisite for future 3-dimensional CEF measurements.
Background
Endothelial cell release of nitric oxide in response to some stressors results in arterial dilatation and is a defining characteristic of healthy vascular tissue. Endothelial dysfunction manifests as impaired dilatation and is a marker for sub-clinical disease, an independent predictor of atherosclerotic progression and adverse cardiac events, and a potential target for medical interventions.1–6 Coronary artery endothelial function (CEF) could historically only be measured with invasive catheterization-based testing,1,7 which precluded studies in stable and low-risk subjects. However CEF can now be quantified noninvasively using 2-dimensional (2D) cine magnetic resonance imaging (MRI) in combination with isometric handgrip exercise8 and the vascular responses are nitric oxide-dependent, reproducible, and impaired in coronary artery disease patients.8–10 Unfortunately, the reported MRI technique only assesses CEF at just one or two locations in an artery and does not allow assessment of the regional heterogeneity present throughout a diseased coronary artery. The aim of this work is to acquire isotropic 3-dimensional (3D) cine MRI to noninvasively measure lumen size along the entire proximal and mid segments of the coronary arteries. Previous ECG-triggered 3D approaches failed because of heart rate changes during exercise. Here we propose a 3D cine acquisition with retrospective gating to cope with exercise-induced heart rate changes. Recent developments in MRI have shown promise for high acquisition acceleration in dynamic imaging with golden-angle rotated non-Cartesian k-space trajectories and compressed-sensing type sparse parallel-imaging reconstructions.11–15 Therefore, we developed and tested the combination of a Golden-Angle Spiral Sparse Parallel-imaging (GASSP) acquisition with a sparse parallel-imaging reconstruction to keep the acquisition duration within a single breath-hold enabling 3D acquisitions during exercise.Methods
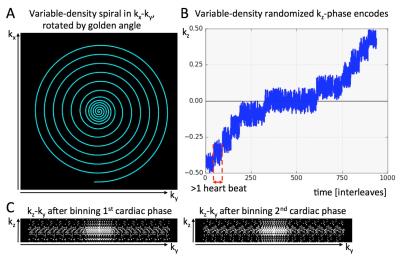
Acquisition: All data were acquired on a 3T scanner (Philips Achieva) with a 32-channel cardiac coil. The sequence was tested in a resolution phantom (with 4.5mm diameter rods) and six datasets were acquired along the right coronary arteries of 3 subjects. Variable-density spiral interleaves (Figure 1A) were acquired for ~20s while rotating consecutive interleaves by the golden-angle (137.508°). Pseudo-randomized phase-encoding was performed in slice direction with a higher density at the k-space center (Figure 1B). The ECG-signal was recorded and used for retrospective data binning to 20 cardiac phases. Example kz-ky sampling pattern for two cardiac phases after retrospective binning is shown in Figure 1C. Imaging parameters were: field-of-view = 300x300x40mm3, slice-oversampling = 25%, water selective spectral-spatial excitation with an excitation angle = 20°, isotropic voxel sizes from 1.1 to 1.3mm, repetition time / echo time = 22 / 2.2 ms. The 3D volume was planned orthogonal to an axial, low-resolution whole-heart coronary MR angiogram covering the proximal and mid right coronary artery. All data were acquired in single 20s breath-holds.
Reconstruction: Data were reconstructed off-line on a MacPro using GPI.16 The GRASP14 reconstruction, originally designed for 2D radial trajectories, was adapted for 3D spiral trajectories and is referred to as GASSP reconstruction. It iteratively minimizes the combination of parallel-imaging data consistency and temporal total-variation based sparsity constraints. After reconstruction, each image slice was deblurred with a single frequency to correct the area around the coronary artery only.17
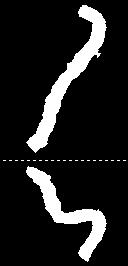
Data Analysis: Cross-sectional area along the coronary arteries was quantified from the 3D data using a semi-automated tool, which performs centerline tracking and a full-width-at-half-maximum (FWHM) segmentation between two manually selected endpoints.18 Results were averaged over 4 mm segments throughout each artery.
Results
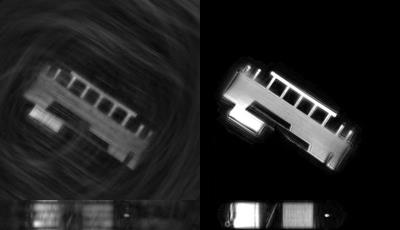
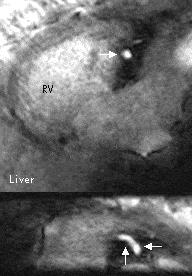
GASSP image reconstruction with 40 iterations took 6-8 hours. Figure 2 shows the phantom data reconstructed (A) with simple gridding and (B) with GASSP. The gridding only reconstruction in Figure 2A shows that the golden-angle rotation and randomized z-encoding ensure undersampling-artifacts that vary for the different cardiac phases enabling sparse reconstruction. The GASSP reconstructed images show little temporal variation and sharp rods approximately the size of a coronary artery. Figure 3 shows an exemplary right coronary artery. Semi-automated centerline tracking detected an average vessel length of 68±14mm in the 6 datasets. An example FWHM segmentation is shown in Figure 4 of the coronary shown in Figure 3. Average vessel area of the 6 vessels was 8.5±2.5mm2.Discussion and Conclusion
A novel high-resolution, isotropic 3D coronary cine MRI breath-hold acquisition and reconstruction scheme was developed. Preliminary data show the potential of this technique to measure coronary vessel area throughout the proximal and mid coronary arteries. Further optimizations are warranted and the method needs to be tested while the subject exercises.Acknowledgements
NIH R01-HL61912-11, R01-HL120905-01, and R01-HL125059-01References
1. Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical Vasoconstriction Induced by Acetylcholine in Atherosclerotic Coronary Arteries. N Engl J Med. 1986;315:1046–1051.
2. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954.
3. Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906.
4. Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KRA, Quyyumi AA. Prognostic Value of Coronary Vascular Endothelial Dysfunction. Circulation. 2002;106:653–658.
5. Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial Effects of Cholesterol-Lowering Therapy on the Coronary Endothelium in Patients with Coronary Artery Disease. N Engl J Med. 1995;332:481–487.
6. Pugh CJA, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, Cable NT, Jones H, Cuthbertson DJ. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307:H1298–1306.
7. Veerasamy M, Bagnall A, Neely D, Allen J, Sinclair H, Kunadian V. Endothelial Dysfunction and Coronary Artery Disease: A State of the Art Review. Cardiol Rev. 2014;1.
8. Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:1657–1665.
9. Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schär M, Gerstenblith G, Stuber M, Weiss RG. Coronary vasomotor responses to isometric handgrip exercise are primarily mediated by nitric oxide: a noninvasive MRI test of coronary endothelial function. Am J Physiol Heart Circ Physiol. 2015;308:H1343–1350.
10. Hays AG, Stuber M, Hirsch GA, Yu J, Schär M, Weiss RG, Gerstenblith G, Kelle S. Non-Invasive Detection of Coronary Endothelial Response to Sequential Handgrip Exercise in Coronary Artery Disease Patients and Healthy Adults. PLoS ONE. 2013;8:e58047.
11. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26:68–76.
12. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–1195.
13. Gamper U, Boesiger P, Kozerke S. Compressed sensing in dynamic MRI. Magn Reson Med. 2008;59:365–373.
14. Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72:707–717.
15. Yerly J, Ginami G, Nordio G, Coristine AJ, Coppo S, Monney P, Stuber M. Coronary endothelial function assessment using self-gated cardiac cine MRI and k - t sparse SENSE: Coronary Endothelial Function Assessment Using Self-Gated Cardiac Cine MRI. Magn Reson Med. 2015;
16. Zwart NR, Pipe JG. Graphical programming interface: A development environment for MRI methods. Magn Reson Med. 2015;74:1449–1460.
17. Noll DC, Pauly JM, Meyer CH, Nishimura DG, Macovski A. Deblurring for non-2D Fourier transform magnetic resonance imaging. Magn Reson Med. 1992;25:319–333.
18. Soleimanifard S, Schar M, Hays AG, Weiss RG, Stuber M, Prince JL. Vessel centerline tracking and boundary segmentation in coronary MRA with minimal manual interaction. In: IEEE Int Symp Biomed Imaging. 2012. p. 1417–1420.
Figures