2779
Gradient Echo Derived Multi-Contrast Imaging for Carotid Atherosclerosis Assessment1Philips Research China, Shanghai, People's Republic of China, 2Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, People's Republic of China, 3Vascular Imaging Lab, Department of Radiology, University of Washington, Seattle, WA, United States
Synopsis
In this work, we developed a post processing framework to generate multi-contrast images from a single three dimensional MR scan of multi-echo gradient echo sequence, which can alleviate the registration problem and further improve the scan efficiency with a total scan time of 3min22sec for carotid atherosclerosis imaging. The initial experiments has demonstrated its feasibility for vessel wall delineation and its potential for plaque component characterization.
Introduction
Atherosclerosis is a chronic systemic vessel wall disease that can cause severe cardio- and cerebrovascular events. The multi-contrast magnetic resonance (MR) vessel wall imaging (VWI) has demonstrated its clinical value to characterize different plaque components1. However, this sequential multi-contrast VWI strategy suffers from scan inefficiency and misregistration problem. Previous studies2,3 have significantly improved the registration issues by using the interleaved multi-contrast VWI within a single MR scan, but the reduction of overall scan time is relatively limited which might cause the intra-scan motion problem. Recently, it has been demonstrated that the multi-echo gradient echo sequence allows simultaneous generation of naturally co-registered multi-contrast images using image post processing methods, including T1 weighted image, R2* (1/T2*) map and susceptibility weighted image4. Therefore, a single scan of this multi-echo gradient echo sequence has the potential to characterize various plaque components (e.g. intra-plaque hemorrhage, calcification)5-7, which can almost eliminate the image registration issue (motion within several delta TEs) while significantly reduce the overall scan time.Aim
To develop a post processing framework for derivation of multi-contrast images from a single MR scan of multi-echo gradient echo sequence and investigate its feasibility for carotid atherosclerosis imaging in this preliminary study.Methods
MR imaging
All of the subjects including 3 healthy volunteers and 3 patients were scanned at Philips Achieva TX 3.0T MR scanner. The scan parameters for three dimensional (3D) multi-echo gradient echo sequence include FA = 15, TE1/delta TE/TR = 3.9/3.5/30ms, 5 echoes, FOV = 200x200x54mm3, resolution = 0.6x0.6x2.0mm3, water excitation RF pulse for fat signal suppression and an overall scan time of 3min22sec. In addition, the conventional two dimensional (2D) T1 and T2 weighted turbo spin echo (TSE) carotid VWI were applied as a reference for plaque component characterization with FOV = 140x140x32mm3, resolution = 0.6x0.6x2.0mm3, TE/TR = 10/800ms (for T1 TSE), TE/TR = 50/4000ms (for T2 TSE) and the total scan time of 5min7sec (for T1 TSE) and 2min40sec (for T2 TSE).
Image post processing
3 different image contrast datasets can be derived from the acquired single 3D volume dataset, including a bright blood T1 weighted image, a black blood R2* map and a susceptibility map. The T1 weighted image and R2* map were estimated from the multi-echo magnitude image datasets using the nonlinear least square fitting for the mono exponential T2* decay model and the susceptibility map was derived from the multi-echo phase image datasets via fieldmap estimation8 and total field inversion9 with projection onto dipole field (PDF) regularization10 for background field removal.
Results
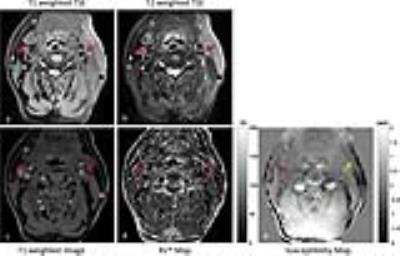
For the results of volunteer scan in figure 1, the vessel wall boundary of common carotid artery can be clearly be delineated on all of the generated image contrasts as shown by the red arrows. However, the estimated susceptibility map was not sufficiently smooth due to the strong variation of susceptibilities (e.g. fat, bone and air, indicated by the yellow arrow). For the results of patient scan in figure 2, the calcification on both sides of internal carotid arteries can be identified by the proposed method and conventional multi-contrast VWI. But it’s still challenging to accurately indicate all of the calcification lesions (left side as shown by the yellow arrow) on the susceptibility map in a complex tissue environment.Discussion and Conclusion
In this work, we presented a post processing framework to generate multi-contrast images from a single MR scan of multi-echo gradient echo sequence, which can be more robustly co-registered to alleviate the registration problem and further improve the scan efficiency to reduce the scan time as 3min22sec for carotid atherosclerosis imaging. In the preliminary experiments, it has demonstrated its feasibility to delineate carotid vessel wall and its potential to characterize plaque components. Further optimization on fat suppression/separation is demanded for accuracy improvement of susceptibility maps.Acknowledgements
No acknowledgement found.References
1. Yuan C, Mitsumori LM, Beach KW, et al. Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology. 2001;221(2):285-299.
2. Fan Z, Wei Y, Xie Y, et al. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. Journal of Cardiovascular Magnetic Resonance. 2014;16(1):1195-1197.
3. Chen S, Zhou Z, Chen H, et al. 3D Large Coverage Atherosclerosis Plaque Assessment within Single Scan (APASS): Preliminary Application in Carotid Artery and Femoral Artery. ISMRM 2015, p2654.
4. Luo J, Jagadeesan BD, Cross AH, et al. Gradient echo plural contrast imaging--signal model and derived contrasts: T2*, T1, phase, SWI, T1f, FST2*and T2*-SWI. Neuroimage. 2012;60(2):1073-1082.
5. Yang Q, Liu J, Barnes SR, et al. Imaging the vessel wall in major peripheral arteries using susceptibility-weighted imaging. J Magn Reson Imaging. 2009;30(2):357-65.
6. Liu Q, Fan Z, Yang Q, et al. Peripheral arterial wall imaging using contrast-enhanced, susceptibility-weighted phase imaging. J Comput Assist Tomogr. 2012;36(1):77-82.
7. Wang C, Liu S, Buch S, et al. Quantitative Susceptibility Mapping of Atherosclerosis in Carotid Arteries. ISMRM 2016, p2552.
8. Liu T, Wisnieff C, Lou M, et al. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med. 2013;69(2):467-476.
9. Liu Z, Kee Y, Zhou D, et al. Preconditioned total field inversion (TFI) method for quantitative susceptibility mapping. Magn Reson Med. 2016. Epub.
10. Liu T, Khalidov I, de Rochefort L, et al. A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed. 2011;24(9):1129-1136.
Figures