2776
Molecular Magnetic Resonance Imaging of Vascular Myeloperoxidase Activity for the Identification of Unstable Atherosclerotic Plaque1Vascular Biology Division, Victor Chang Cardiac Research Institute, Sydney, NSW, Australia, 2Biological Resources Imaging Laboratory, University of New South Wales, Sydney, Australia, 3Department of Physics, Centre for Beam Applications, National University of SIngapore, Singapore, Singapore
Synopsis
Non-invasive imaging of unstable atherosclerotic plaques prone to rupture remains an unmet need in clinical cardiology. This is because current imaging modalities provide information on plaque burden, luminal stenosis and calcification, whereas they have limited ability to discern unstable from stable plaques. We used the tandem stenosis mouse model of unstable plaque in conjunction with MRI and a sensor (bis-5-hydroxytryptamide-DTPA-Gd) for the activity of the inflammatory enzyme myeloperoxidase. Our results reveal sustained and greater enhancement of unstable experimental plaque with the targeted sensor compared with its non-targeted analog (DTPA-Gd), and these results were confirmed by separate biochemical and histological analyses.
Introduction
Current non-invasive vascular imaging modalities have limited capacity to discern stable from unstable atherosclerotic plaque. Inflammation is a key driver of atherosclerotic plaque instability and acute rupture, which is the precipitating event for the majority of acute myocardial infarctions (AMI). Myeloperoxidase (Mpo) is an inflammatory enzyme released by macrophages and neutrophils that is implicated in plaque destabilization and that correlates with adverse clinical outcomes1. We therefore aimed to investigate whether magnetic resonance imaging of arterial myeloperoxidase activity can be used for the detection of unstable atherosclerotic plaque. To achieve this, we used the recently described tandem stenosis (TS) mouse model of unstable plaque2.Methods
TS surgery was performed in male C57BL/6J Apoe–/– (n=9) or Mpo–/–Apoe–/– (n=3) mice (at 12w age) fed a Western diet. The surgery involved placement of two non-occlusive sutures around the right common carotid artery (3mm apart) with the distal suture adjacent to the bifurcation. At 7-weeks post-operation, anesthetized mice underwent magnetic resonance imaging using a 9.4 T Bruker Biospec 94/20 with a 50mm quadrature RF coil. Mice were imaged before and after intravenous administration of the Mpo sensor bis-5-hydroxytryptamide-DTPA-Gd (Mpo-Gd, n=5) or its control contrast agent (DTPA-Gd, n = 5). Active Mpo oxidizes Mpo-Gd resulting in oligomerization of the sensor. Two-dimensional time-of-flight (TOF; FLASH, TR 34ms, TE 3ms, ETL 96, slice thickness 0.5mm, FOV 20 x 20mm, 128 x 128 data matrix), T2-weighted (TurboRARE, TR 2500ms, TE 43ms, ETL 8, slice thickness 1mm, FOV 20 x 20mm) and serial T1-weighted (TurboRARE, TR 1500ms, TE 8.5ms, ETL 8, slice thickness 1mm, FOV 20 x 20mm, 192 x 192 data matrix) images were acquired. OsiriX (Pixmeo, Switzerland) was used for image analysis. In T1-weighted images, ROIs were assigned to the vessel wall, skeletal muscle and background to assess signal intensity (SI) with calculation of the contrast-to-noise ratio (CNR) as follows: CNR = (SIVessel wall – SIskeletal muscle)/SIbackground. In addition, arterial Mpo activity was determined using a mass spectrometry-based method recently described3. The mice were then culled and tissue collected for histological characterization of plaque type and gadolinium determination by nuclear microsocopy4. The Kruzkal-Wallis one-way analysis of variance was used to analyse the data.Results
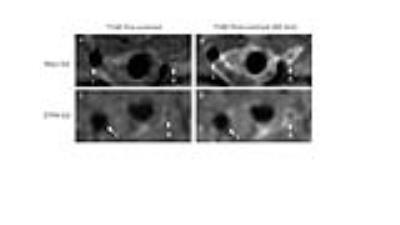
Administration of Mpo-Gd resulted in sustained enhancement of atherosclerotic plaque in the right common carotid artery at 60 min (CNR 21 ± 1.7, mean ± SEM) with minimal enhancement of the non-diseased left common carotid artery (2.9 ± 1, n=5, p <0.05) (Figure 1A,B). At 60 min post-contrast injection, the enhancement of unstable plaque was greater with Mpo-Gd than the non-targeted DTPA-Gd (24.5 ± 2.3 vs 13.4 ± 0.8, p < 0.05) (compare Fig. 1B with 1D). Tissue elemental analysis by nuclear microscopy confirmed higher concentrations of gadolinium in unstable plaque following administration of Mpo-Gd compared with DTPA-Gd (125.5 ± 18.6 ppm/g vs 26 ± 16.1 ppm/g). Plaque enhancement following administration Mpo-Gd to Mpo–/–Apoe–/– mice was not significantly different to non-targeted DTPA-Gd (n=3, CNR 9.4 ± 2.3) confirming Mpo-dependence for tracer activation. The enhancement of unstable plaque with Mpo-Gd (ΔCNR 16 ± 2.1) was 1.7-fold greater than that of stable plaque in the brachiocephalic trunk (ΔCNR 9.5 ± 1.9). In vivo (0.034 ± 0.04 fmol/wwt, n=5) and ex vivo (49.24 ± 43.95 pmol/mgp, n=5) analysis of tissue Mpo activity confirmed higher enzymatic activity in unstable compared with stable plaque (aortic arch, where no activity was detected).Discussion
The results demonstrate that molecular magnetic resonance imaging of Mpo activity yields enhancement of experimental atherosclerotic plaque compared with non-diseased arteries. This enhancement is greater for unstable compared with stable plaque. As the inflammatory enzyme Mpo is implicated in atherosclerotic disease progression in humans, molecular imaging with Mpo-Gd may provide specific information about disease activity that is not obtained by current vascular imaging modalities that predominantly focus on the assessment of plaque volume, luminal stenosis and calcification. Selective enhancement of atherosclerotic plaque by Mpo-Gd has the potential to improve the quantification of plaque burden in humans by coronary magnetic resonance angiography, where thresholds of enhancement may allow further delineation of unstable plaque and therefore high-risk patients.Conclusion
Molecular magnetic resonance imaging of Mpo activity enhances experimental atherosclerotic plaque to an extent that correlates with features of plaque instability. This imaging strategy could facilitate clinical coronary magnetic resonance angiography for the identification of high-risk patients.Acknowledgements
St Vincent's Clinic FoundationReferences
1. Zhang R et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 286:2136-2142 (2001).
2. Chen Y-C et al. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ Res 113:252-265 (2013).
3. Talib et al. Detailed protocol to assess in vivo and ex vivo myeloperoxidase activity in mouse models of vascular inflammation and disease using hydroethidine. FRBM 97:124-135 (2016).
4. Rajendran R et al. Nuclear microscopy: a novel technique for quantitative imaging of gadolinium distribution within tissue sections. Microsc Microanal 15:338-44 (2009).
Figures