2775
Robust fat suppressed direct thrombus imaging (MPRAGE) sequence with a large field-of-view at 3T1Kumamoto Chuo Hospital, Kumamoto, Japan, 2Philips Electronics Japan, Tokyo, Japan
Synopsis
MR plaque imaging is promising for characterizing atherosclerotic plaque. We proposed direct thrombus imaging (MPRAGE), based on mDIXON turbo field-echo sequence, could provide more robust and stable black-blood imaging with a large field-of-view compared with conventional sequences.
Purpose
MR plaque imaging is promising for characterizing atherosclerotic plaque. Recently, various techniques have been proposed to assess the atherosclerotic plaque1-3. Direct thrombus imaging based on magnetization prepared rapid gradient echo (MPRAGE) is one of the most important method for evaluating the atherosclerotic plaque1. Otherwise, 3D T1-TSE sequence is used for the plaque imaging3. Such atherosclerotic plaque with a large field-of-view (FOV), from aortic artery to internal carotid artery, is clinically desirable. However, current methods often suffer from poor fat suppression because of B0 inhomogeneity. In this study we tried to use the 3D MPRAGE direct thrombus imaging with a large FOV (neck and chest area) with more robust fat suppression by using improved DIXON-based gradient echo sequence (mDIXON-XD). The purpose of this study was to optimize the imaging parameters for mDIXON-MPRAGE and to evaluate the feasibility by comparison of conventional sequences.Methods
mDIXON direct thrombus imaging (MPRAGE) sequence: mDIXON XD is promising for application to head-neck area with higher robustness of fat suppression with a large FOV4,5. mDIXON-MPRAGE sequence consists of mDIXON XD segmented gradient echo sequence (T1-turbo field echo: T1TFE) and non-volume-selective inversion recovery (IR) pulse (Fig.1).
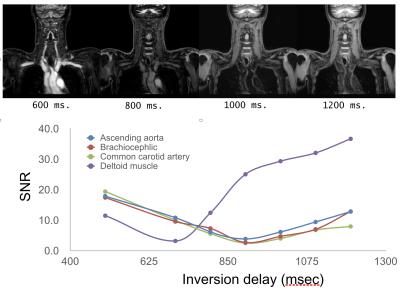
Experiments: A total of 7 volunteers and 8 patients were examined on a 3.0T whole-body clinical system (Ingenia, Philips Healthcare). The study was approved by the local IRB, and written informed consent was obtained from all subjects. (1) Parameter optimization: To investigate an adequate inversion delay (TI) for nulling blood signals, we found out the optimal TI (500,600 … 1400 and 1500ms). To quantitatively compare the effect of respective invert time, we measured the signal-to-noise-ratio (SNR) by the signal of the several regions of interest. Also images of respective TIs were visually assessed and compared by the radiologist and technologists. (2) Comparison with conventional sequence: To demonstrate the feasibility of mDIXON-MPRAGE, we calculated the signal intensity ratio (SIR) of the hemorrhagic plaque and muscle in the patients. Subsequently, we compared visually the image quality with conventional 3D T1-TSE sequence (routinely acquired in our institute) in regards to overall SIR and the presence of artifacts. Imaging parameters for mDIXON-MPRAGE were; Coronal, voxel size=1.00x1.00mm2, Thickness=1.0mm, shot interval=3000ms, TR=4ms, TE1/TE2=1.38/2.6ms, flip angle=10°, NSA=1, TFE factor=100 and total acquisition time=3m39s. On the other hand, imaging parameters for 3D T1-TSE BBI were; Coronal, voxel size=1.0x1.0mm2, Thickness=1mm, TR=400ms, TE=1.6ms, flip angle=75°, refocusing angle=30°, NSA=2, TSE factor=20, SPAIR fat suppression and total acquisition time=4m36s.
Results and Discussion
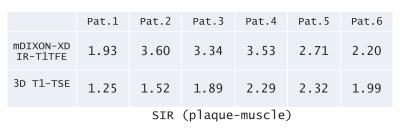
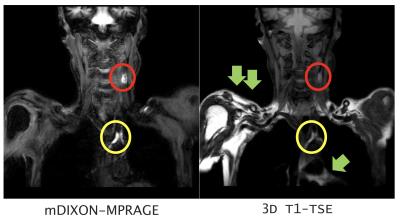
In parameter optimization, the null point of blood signals was around 900~1000ms. (Figure 2). In the comparison of mDIXON-MPRAGE and 3D T1-TSE sequence, SIR (plaque-muscle) of mDIXON- MPRAGE is significantly higher than that of 3D T1-TSE (Table 1). Moreover, 3D T1-TSE images showed failed fat suppression in all cases. mDIXON-MPRAGE could provide more robust and stable images without any artifacts and failed fat suppression. Representative black-blood images with large FOV are shown in Figure 3.Conclusion
The optimized mDIXON-MPRAGE showed good SNR, sufficient blood suppression and robust fat suppression with the large FOV from aortic artery to internal carotid arteries. Although further clinical investigation is needed, the optimized mDIXON- MPRAGE would be promising for evaluating the atherosclerotic plaque with larger FOV including head and chest area.Acknowledgements
No acknowledgement found.References
1. Moody AR, Murphy RE, Morgan PS, et al: Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 107:3047–3052, 2003
2. Yoshida K, Narumi O, Chin M, et al: Characterization of carotid atherosclerosis and detection of soft plaque with use of black-blood. AJNR Am J Neuroradiol 29: 868–74, 2008
3. Yamada N, Higashi M, Otsubo R, et al: Association between signal hyperintensity on T1-weighted MR imaging of carotid plaques and ipsilateral ischemic events. Am J Neuroraidol 28: 287–292, 2007
4. Yoneyama M, et al. Non-Contrast, Flow-Independent, Relaxation-Enhanced Subclavian MR Angiography Using Inversion Recovery and T2 Prepared 3D Gradient-Echo DIXON Sequence. Proc. ISMRM 2016:2252
5. Toyonari N, et al. Non-Contrast Gate-Free Aortic MR Angiography with Robust Fat Suppression. Proc. ISMRM 2016:2684
Figures