2774
3D black-blood DCE-MRI using radial stack-of-stars acquisition and CS reconstruction: application in carotid and femoral arteries1Department of Biomedical Engineering and Physics, Academic Medical Center, Amsterdam, Netherlands, 2Department of Vascular Medicine, Academic Medical Center, Amsterdam, Netherlands, 3Department of Radiology, Academic Medical Center
Synopsis
We implemented a high temporal resolution 3D black-blood protocol for DCE imaging of atherosclerotic plaques using a combination of a radial stack-of-stars sampling scheme, motion-sensitised (iMSDE) blood suppression and CS reconstruction. Using this approach, 3D black-blood DCE images with a temporal resolution of 12s could be obtained. In this work, we show the application of our method in patients with femoral and carotid artery plaques.
Introduction
Dynamic contrast-enhanced imaging of the vessel wall is a powerful tool in the characterization of atherosclerotic plaques. However, this technique ideally requires high spatiotemporal resolution, while at the same time achieving adequate blood signal suppression. While 2D black-blood DCE [1,2] and 3D bright blood DCE [3] implementations have been shown, the combination of these two has has not been reported so far. In this work, we present a 3D black-blood DCE MRI protocol, based on a radial stack-of-stars sampling scheme, motion-sensitized blood suppression and CS reconstruction. We show the feasibility of this approach in carotid or femoral arteries of patients with atherosclerosis.Methods
Sequence design: We implemented a tiny golden angle radial stack-of-stars sampling scheme [4,5]. This was combined with a 3D TFE sequence with motion-sensitized (iMSDE) black-blood pre-pulse [6] (Figure 1). Every TFE-shot acquired exactly one z-stack of radial spokes. The angles for subsequent TFE shots were varied using a tiny golden angle increment of 38.97 degrees. A low-high sampling order in the kz-direction, as well as choosing a low TFE factor assured effective blood suppression. Additionally, frequency selective fat suppression was applied. Specific sequence parameter were as follows: TE=3.2ms, TR=7.2ms, TFE-factor 36; FA=15°; FOV= 250x250x60 mm3 (femoral), FOV=120x120x50 mm3 (carotid); resolution= 0.7x0.7x2 mm3 (femoral); resolution=0.7x0.7x1.4 mm3 (carotid). The temporal resolutions are 11.9s and 11.8s per frame for the femoral and carotid scan respectively.
Image Reconstruction: Slices were reconstructed in parallel, after inverse FFT of the k-space in the kz-direction. A CS reconstruction was performed with a temporal TGV-constraint [7]. Sensitivity maps were estimated from the k-space using the ESPIRiT method [8].
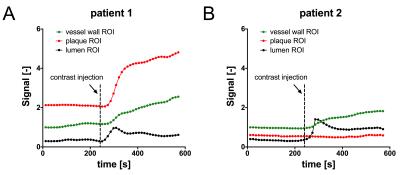
Patient scans and analysis: Images were acquired at 3T (Philips Ingenia) using a 16-channel anterior coil (femoral) and 8-channel carotid coil. After scout scans to locate the vessel regions of interest, 3D DCE scans were acquired continuously for 10 minutes. Four minutes after the start of the scan, a Gd-based contrast agent (Gadovist; 0.1 mmol/kg) was injected intravenously. A time-intensity analysis of a femoral scan was used to illustrate differences in dynamic contrast enhancement between ROIs taken from healthy vessel wall, plaque and the lumen.
Results & Discussion
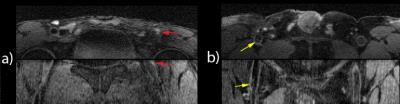
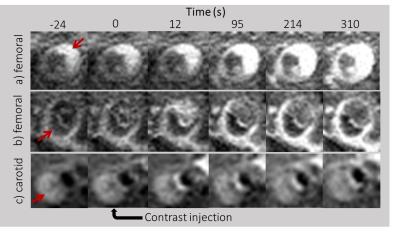
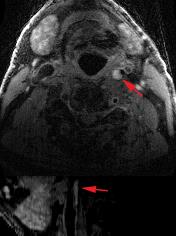
Figure 2a and 2b show dynamic femoral scans of two patients. Corresponding signal intensity-time curves for different ROIs are shown in Figure 3. Figure 4a and 4b show static close-ups of the femorals of the same patients. High perfusion in the plaque of femoral patient A is apparent from the steep contrast uptake, which differs significantly from the uptake curve shown for the vessel wall. On the other hand, femoral patient B shows a plaque lacking significant contrast uptake, indicative of a large lipid core. The strong hypointense pre-contrast signal may well be explained by repetitive use of the T2-preparation in combination with short TFE shots, saturating the low T2 lipid core. Note that the healthy vessel wall signal has a comparable rate of increase for the two femoral patients. While the lumen signal in patient B increases slightly more as compared to patient A, it does not cause serious flow artifacts or signal bleed into the surrounding tissue. Also, in case of the carotid protocol (Fig 3C & 5) very good blood suppression was obtained, and strong enhancement of a fibrous cap between the plaque and lumen was observed.Future directions
To fulfill the demands for a high temporal resolution and sufficient blood suppression, an isotropic resolution was not feasible at the moment. Further improvements in the CS reconstruction pipeline might improve upon this. Finally, because 3D black-blood DCE scans highly complicate proper sampling of the AIF, we will combine our approach with pre-bolus AIF measurements to obtain pharmacokinetic parameters of the plaques.Conclusion
We have shown the feasibility of 3D black-blood dynamic contrast enhancement in carotid and femoral arteries. Spatiotemporal resolution and blood suppression were sufficient to assess differences in enhancement patterns between healthy vessel wall and plaque and between different types of plaque. We believe our method can be a powerful addition to current MR protocols for characterization of atherosclerotic plaques.
Acknowledgements
No acknowledgement found.References
1. Wu, Tingting, et al. "Homologous black-bright-blood and flexible interleaved imaging sequence (HOBBI) for dynamic contrast-enhanced MRI of the vessel wall." Magnetic resonance in medicine 73.5 (2015): 1754-1763.
2. Calcagno, Claudia, et al. "SHILO, a novel dual imaging approach for simultaneous HI-/LOw temporal (Low-/Hi-spatial) resolution imaging for vascular dynamic contrast enhanced cardiovascular magnetic resonance: numerical simulations and feasibility in the carotid arteries." Journal of Cardiovascular Magnetic Resonance 15.1 (2013): 1.
3. Mendes, Jason, et al. "Three-dimensional dynamic contrast enhanced imaging of the carotid artery with direct arterial input function measurement." Magnetic resonance in medicine 72.3 (2014): 816-822.
4.Feng, Li, et al. "Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI." Magnetic resonance in medicine 72.3 (2014): 707-717.
5.Wundrak, Stefan, et al. "A small surrogate for the golden angle in time-resolved radial MRI based on generalized fibonacci sequences." IEEE transactions on medical imaging 34.6 (2015): 1262-1269.
6.Wang, Jinnan, Vasily L. Yarnykh, and Chun Yuan. "Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence." Journal of Magnetic Resonance Imaging 31.5 (2010): 1256-1263.
7.Knoll, Florian, et al. "Second order total generalized variation (TGV) for MRI." Magnetic resonance in medicine 65.2 (2011): 480-491.
8.Uecker, Martin, et al. "ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA." Magnetic resonance in medicine71.3 (2014): 990-1001.
Figures