2773
Assessment of aortic pulse wave velocity in patients with peripheral artery disease1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Anesthesiology, Stony Brook University, Stony Brook, NY, United States
Synopsis
Pulse wave velocity (PWV) provides a measure of arterial stiffness by quantifying the propagation velocity of the systolic pressure wave along the arterial wall. Prior studies have reported that PWV is elevated in patients with peripheral artery disease (PAD), and here we sought to determine whether the elevation in PWV is correlated with PAD disease severity as measured by the ankle-brachial index (ABI). No correlation was observed between ABI and PWV, suggesting that the systemic nature of atherosclerosis may be contributing to the increase in PWV more than the specific lesions that result in a reduction in ABI.
Purpose
Pulse wave velocity (PWV) is a measure of arterial stiffness equal to the propagation velocity of the systolic pulse wave along a segment of the arterial tree. Elevated PWV has been shown to correlate with aging1-3 and is also independently associated with cardiovascular disease4, making PWV an important marker of vascular health. It was previously reported that patients with peripheral artery disease (PAD), an atherosclerotic disease of vessels supplying the legs, had elevated PWV in the aortic arch compared to their age-matched peers5. However it is unclear whether the elevation is due to the systemic nature of atherosclerosis, or if it is specifically related to the macrovascular lesions that define PAD. PAD disease presence and severity is clinically characterized by the ankle-brachial index (ABI), the ratio of blood pressure in the ankle and brachial artery. Here, we measured PWV in the aortic arch and descending aorta with MRI in patients with varying degrees of peripheral artery disease severity to investigate whether an association exists between ABI and PWV.Methods
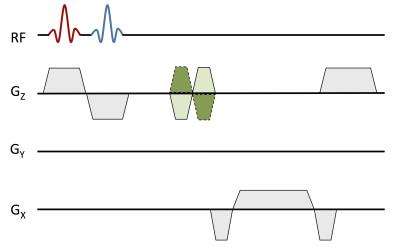
By measuring the transit time (Δt) of the systolic wave front between two locations in the aorta and the path length (Δd) separating the two measurement sites, PWV can be quantified as: PWV= Δd/Δt (m/s). The arrival of the systolic wave can be measured with MRI using velocity-encoded projections6. By removing phase encoding from a traditional 2D phase contrast sequence and choosing a suitable readout direction to avoid vessel overlap in the projection image, velocity can be measured with temporal resolution equal to twice the TR (temporal resolution = 7.4 - 12 ms, depending on segment) (Figure 1). For the aortic arch, the ascending and proximal descending aorta were captured in a single slice7. The descending aorta segment required signal acquisition at two separate locations3 (Figure 2). There, back-to-back RF pulses were used to excite the two slices within the same TR, and localized coil sensitivities were used to separate the signal readout from the two locations. In both segments, the complex difference between positive and negative flow-encoded projections was then averaged in a region corresponding to the vessel (which was verified in a fully encoded reference image), yielding a waveform that is proportional to velocity. The onset of the systolic wave at each location was determined based on intersecting tangents, and the temporal offset was determined based on the foot-to-foot method8. Descending aorta path length was determined from a set of axial scout images, and aortic arch path length was determined based on an oblique coronal image, bisecting the aortic arch in profile.
65 patients (67±8 years old) with PAD were recruited to participate in the study. ABI was measured bilaterally prior to participation in the MRI study, and was recorded as the lower of the two measurements. During the MRI scan, PWV was measured in the aortic arch and descending aorta segments. Pulse sequence parameters include: Arch segment: FOV = 256×448 mm2, slice thickness = 10 mm, acquired matrix = 88×1 (reference image acquired with matrix = 88×224), TR/TE = 3.7/1.7 ms, VENC = 110 cm/s; descending aorta segment: FOV = 352×353 mm2; slice thickness = 10 mm, acquired matrix = 256×1 (reference image acquired with matrix = 256×256), TR/TE = 6.0/4.0 ms, VENC = 125 cm/s.
Results
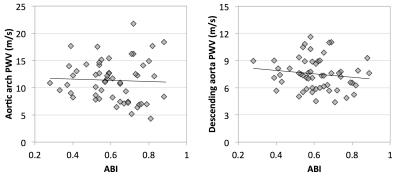
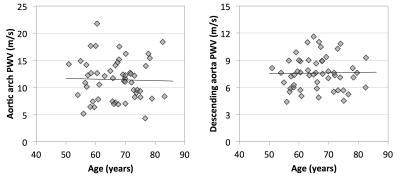
PWV was successfully measured in 55 and 56 patients for the arch and descending aorta, respectively. The average PWV for all subjects was 11.3±3.7 m/s in the aortic arch and 7.8±2.4 m/s in the descending aorta. Figure 3 shows scatter plots of ABI and PWV in both of the arterial segments. No significant correlations were observed between ABI and PWV. Correlation coefficient was -0.04 and -0.14 in the arch and descending aorta, respectively (p>0.05 for both), however several outliers may be impacting the correlation. Age was not controlled for, however a correlation was not observed between age and PWV in this patient population (Figure 4).Discussion & Conclusions
Despite the fact that significant correlations were not observed, the average PWV was elevated compared to older subjects enrolled in previous studies, which measured PWV as 8.1±0.4 m/s (arch)5 and approximately 6 m/s (descending aorta)3. Our results suggest that the systemic burden of atherosclerosis may be independent of the lesions that result in a decrease in ABI. Furthermore, the lack of association between age and PWV may suggest that the presence of atherosclerotic disease is affecting PWV more than the aging process. Additional analysis will be completed to investigate whether there is an association between PWV and other factors such as diabetes, and smoking pack-year history.
Acknowledgements
Supported by an award from the American Heart Association and NIH Grants R01 HL075649, HL109545, and T32 HL007954.References
1. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245.
2. The Reference Values for Arterial Stiffness' Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values.” European Heart Journal. 2010;31:2338–2350.
3. Langham MC, Li C, Wehrli FW. Non-triggered quantification of central and peripheral pulse-wave velocity. Journal of Cardiovascular Magnetic Resonance. 2011;13:81.
4. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. JACC. 2010;55:1318–1327.
5. Langham MC, Englund EK, Mohler ER III, Li C, Rodgers ZB, Floyd TF, Wehrli FW. Quantitative CMR markers of impaired vascular reactivity associated with age and peripheral artery disease. Journal of Cardiovascular Magnetic Resonance. 2013;15:1–10.
6. Langham MC, Jain V, Magland JF, Wehrli FW. Time-resolved absolute velocity quantification with projections. Magn Reson Med. 2010;64:1599–1606.
7. Langham MC, Li C, Magland JF, Wehrli FW. Nontriggered MRI quantification of aortic pulse-wave velocity. Magn Reson Med. 2011;65:750–755.
8. Wentland AL, Grist TM, Wieben O. Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc Diagn Ther. 2014;4:193–206.
Figures