2770
Distribution and burden of atherosclerosis in patients with anterior circulation cerebral ischemic events: Characterization using combined extracranial and intracranial vessel wall MRI1University of Washington, Seattle, WA, United States, 2Tangshan Gongren Hospital, Tangshan, People's Republic of China
Synopsis
Both extracranial and intracranial atherosclerosis may be implicated in large-artery atherothrombotic stroke. This proof-of-concept study characterized the distribution and burden of atherosclerosis in thirteen patients with anterior circulation cerebral ischemic events using combined extracranial and intracranial vessel wall MRI. We found that atherosclerotic plaques were highly prevalent in both extracranial and intracranial carotid arteries. Larger plaque burden measured as plaque index on black-blood vessel wall MRI, rather than luminal stenosis on time-of-flight MRA, was significantly associated with clinical symptoms. Black-blood vessel wall MRI may be useful in identifying the culprit plaque in patients with suspected large-artery atherothrombotic stroke.
Purpose
Large-artery atherothrombotic stroke is a common subtype of ischemic stroke. Both extracranial and intracranial carotid arteries are common sites for atherosclerosis.1 As luminal stenosis is an imperfect marker of lesion severity, the role of atherosclerosis in ischemic stroke based on angiography techniques may be underestimated, particularly for intracranial atherosclerosis of which direct plaque imaging has been limited until recently.2,3 This proof-of-concept study sought to characterize the distribution and burden of atherosclerosis in patients with anterior circulation cerebral ischemic events using combined extracranial and intracranial vessel wall MRI.Methods
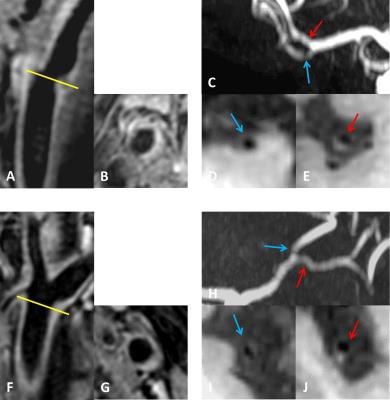
Subjects: We recruited 13 patients with anterior circulation cerebral ischemic events in the past 6 months. Cardioembolic strokes and other rare etiologies diagnosed during clinical workup were excluded. Institutional review board approval and written informed consent were obtained. MR protocol: Subjects were scanned at Philips 3T using an eight-channel carotid coil for extracranial carotid arteries and an eight-channel head coil for intracranial arteries. The carotid protocol included two previously described 3D sequences with 0.8 mm isotropic resolution.4,5 The intracranial protocol included T1-weighted, T2-weighted, and proton-density-weighted volumetric isotropic turbo spin echo6 with 0.6 or 0.8 mm isotropic resolution. Zero-padding was used to reduce pixel size in all three dimensions. Routine time-of-flight MRA (both carotid and brain) and brain MRI were also performed. Image analysis: Images were analyzed blinded to clinical information using a DICOM viewer with multi-planar reconstruction (MPR) views. Readers screened the carotid arterial tree on black-blood images for distinct plaques defined as focal wall thickening that exceeded two times reference wall thickness. Plaque location, luminal stenosis, plaque index (1 - lumen diameter / outer wall diameter), and intraplaque hypointensities (indicating necrotic core or calcification) were recorded. Luminal stenosis was graded on time-of-flight MRA: no stenosis, mild (<50%), moderate (extracranial: 50-70%, intracranial: ≥50% without flow void), severe (extracranial: >70%, intracranial: flow void). Plaque index was measured on MPR images of black-blood MRI that gave a cross-sectional view of the lesion. Statistics: Pearson’s correlation coefficient, McNemar’s test, the paired t-test and the Wilcoxon signed-rank test were used as appropriate to compare the symptomatic and asymptomatic side.Results
Clinical characteristics: Mean age was 63.2 ± 14.9 years. Eight (61.5%) were male. The mean time interval between clinical event and vessel wall MRI was 110±70.3 days. Plaque distribution: Distinct plaques were identified bilaterally in all subjects and frequently affected all three sequential arterial beds (extracranial carotid artery, intracranial carotid artery, terminal branches of internal carotid artery) irrespective of symptom status (Figure 1-2). The most affected arterial segment on the symptomatic side was extracranial carotid artery whereas on the asymptomatic side was middle cerebral artery. However, there was no significant association between plaque distribution and clinical symptom. Plaque burden: The symptomatic and asymptomatic side did not show a difference in the degree of luminal stenosis, although numerically there were more lesions with moderate or severe stenosis on the symptomatic side (Figure 3). Nonetheless, plaque index was significantly higher on the symptomatic side (0.60 ± 0.16 vs. 0.49 ± 0.08, p=0.024). Intraplaque hypointensities were frequently noted in at least one plaque on both symptomatic and asymptomatic side. Correlation between different arterial beds: The correlation in plaque index appeared higher between the two extracranial carotid arteries or between the two intracranial carotid arteries compared to that between ipsilateral extracranial and intracranial carotid arteries (Figure 4).Discussion
In patients with anterior circulation cerebral ischemic events, we found that atherosclerotic plaques were highly prevalent in both extracranial and intracranial carotid arteries. Such extensive atherosclerosis was seen on both sympatomatic and asymptomatic side, suggesting this is mainly driven by systemic risk factors. On the other hand, a significant association was found between focal plaque burden measured as plaque index and clinical symptoms. Therefore, among the multiple plaques that may be present along the carotid arterial tree, those with larger plaque burden may be at higher risk. Despite a high correlation in plaque index between bilateral extracranial carotid arteries and between bilateral intracranial carotid arteries, the correlation between ipsilateral extracranial and intracranial carotid arteries was weak. Plaques with large burden may tend to develop in extracranial carotid arteries for some patients whereas in intracranial carotid arteries for others.Conclusions
Multiple atherosclerotic plaques are commonly seen along the carotid arterial tree in patients with anterior circulation cerebral ischemic events. Detecting distinct plaques in either extracranial or intracranial carotid arteries does not exclude the possibility of culprit plaques located in the other artery bed.Acknowledgements
The authors thank Kristi D. Pimentel and Alice M. Graden for their help with patient recruitment. Grant support is from the National Institutes of Health (R01 NS083503).References
2. Dieleman N, van der Kolk AG, Zwanenburg JJM, Harteveld AA, Biessels GJ, Luijten PR, Hendrikse J. Imaging intracranial vessel wall pathology with magnetic resonance imaging: Current prospects and future directions. Circulation. 2014. 130(2):192-201.
3. Li ML, Xu WH, Song L, Feng F, You H, Ni J, Gao S, Cui LY, Jin ZY. Atherosclerosis of middle cerebral artery: Evaluation with high-resolution MR imaging at 3T. Atherosclerosis. 2009. 204(2):447–452.
4. Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med. 2011. 65(3):627-637.
5. Wang J, Bornert P, Zhao H, Hippe DS, Zhao X, Balu N, Ferguson MS, Hatsukami TS, Xu J, Yuan C, Kerwin WS. Simultaneous noncontrast angiography and intraPlaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med. 2013. 69(2):337-345.
6. Qiao Y, Steinman DA, Qin Q, Etesami M, Schar M, Astor BC, Wasserman BA. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011. 34(1):22-30.
Figures