2750
Cardiomyopathy in later-onset Fabry disease: a correlative study of T1 mapping on MR and histology1Radiology, Taipei Veterans General Hospital, Taipei, Taiwan, 2School of Medicine, National Yang Ming University, Taipei, Taiwan, 3Department of Medical Imaging and Radiological Sciences, National Yang Ming University, Taipei, Taiwan, 4Pathology, Taipei Veterans General Hospital, Taipei, Taiwan, 5Pediatrics, Taipei Veterans General Hospital, Taipei, Taiwan, 6Radiology, Kaohsiung Veterans Genertal Hospital, Kaohsiung, Taiwan, 7GE Healthcare, Taipei, Taiwan, 8GE Healthcare MR Research China, Beijing, China
Synopsis
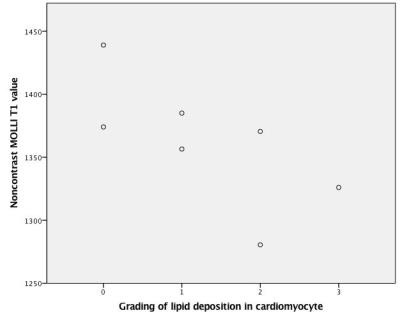
Fabry disease is a rare and X-linked disorder characterized by accumulation of glycosphingolipid within lysosomes and resultant multiple organ damage including heart. Since lipid is known to shorten the MRI parameter T1, non-contrast T1 mapping has emerged as key imaging modality to assess Fabry cardiomyopathy and early detection of lipid deposition. This study provides a histologic validation of 7 male patients of untreated later-onset Fabry disease to demonstrate the negative correlation between native myocardial T1 value and severity of lipid deposition (correlation coefficient, -0.771; p, 0.042) and justifies the application of T1 mapping as a noninvasive predictor of surveillance strategy.
Purpose
Fabry disease (FD), or Anderson-Fabry disease is an X-linked glycolipid storage disorder affecting multiple organs including heart, which is a leading cause of death of these patients. Cardiovascular manifestations of Fabry disease include left ventricular hypertrophy, aortic and mitral regurgitation, conduction defects, coronary artery disease, hypertension, and aortic root dilation. The well-described histologic and electron microscopic findings in Fabry disease cardiomyopathy are hypertrophic vacuolated cells with electron dense concentric lamellar bodies (vacuolization of myocytes). For non-invasive evaluation of Fabry cardiomyopathy, cardiovascular MRI (CMR) has emerged as a key imaging modality to provide both quantitative and qualitative assessment, especially quantitative T1 mapping, which is suggested as a disease-specific imaging biomarker, since lipoid is known to shorten the MR parameter of T1.1,2 Inversion-recovery technique, such as modified Look-Locker Inversion recovery (MOLLI), is an efficient and mature method of measurement of T1 value.3 The purpose of this study is to evaluate the relationship between non-contrast myocardial T1 value and histological grading of vacuolization of myocytes.Material and method
The study population included 7 male patients with FD without enzyme replacement therapy, ages ranging from 37 to 66 years (mean, 51.7y). In all patients, the diagnosis of FD was based on the identification of α-galactosidase A mutations. All these patients underwent cardiovascular MRI on a 3T clinical scanner (GE Healthcare, Milwaukee, USA) before endomyocardial biopsy. In addition to evaluation of cardiac function and late gadolinium enhancement, the MRI protocol included MOLLI sequence (Figure 1) before gadolinium administration to measure myocardial T1 values at the middle layer of septum. Endomyocardial muscle samples were processed for routine histologic analysis including H&E stain and Toluidine blue, and vacuolization of myocyte, and the degree of lipid deposition was graded using a semiquantitative scale (0 to 3) by an experienced pathologist. Each case was scored blinded to clinical information. Spearman rank correlation coefficient was calculated to assess the relationship between T1 value and vacuolization grade.Results
The myocardial T1 values range from 1287 to 1387 ms (1343.6 +/- 35.9 ms). The median score of vacuolization is 1 (range, 0-3). On relationship between T1 mapping values and vacuolization grades, there was a significant negative linear correlation between the T1 values and grading of myocyte vacuolization (p value, 0.042; correlation coefficient, -0.771). (Figure 2)Discussion
FD causes the accumulation of glycosphingolipid in myocytes and histologically the vacuolation and extent of lamellar bodies can be substantial. Several literatures have proposed that this abnormal accumulation will lower the T1 value and not be offset by potential prolongation from fibrosis.4,5 However in some extraction experiments only a few glycosphingolipid are obtained which probably cannot explain the T1 lowering effect from protons on the CH2 groups of lipids.5,6 In our study, we provide histologic validation by the observed negative correlation between T1 values and severity of myocyte vacuolization by light microscopy. The exact mechanisms of decreased T1 value in FD require further work.Conclusion
This study shows that reduced non-contrast myocardial T1 values are linearly correlated with the severity of myocyte vacuolization. Not only the reduced T1 values due to lipid deposition is confirmed, but also that non-contrast T1 mapping may serve as a non-invasive diagnostic modality for prediction of the severity of cardiomyopathy in FD. By using non-contrast T1 mapping, we can non-invasively monitor the severity of cardiomyopathy in these patients and hence more accurately adjust the strategy of surveillance and management for these patients.Acknowledgements
No acknowledgement found.References
1. Richard T, Kelvin C, Aneal K, et al. T1 Mapping With Cardiovascular MRI Is Highly Sensitive for Fabry Disease Independent of Hypertrophy and Sex. Circ Cardiovasc Imaging. 2013;6:637-645.
2. Daniel S, Steven W, Stefan P, et al. Identification and assessment of Anderson-Fabry Disease by cardiovascular MR noncontrast myocardial T1 Mapping. Cir Cardiovasc Imaging. 2013;6:392-398.
3. Akos VS, Giuseppe M, U. Joseph S, et al. Overview of myocardial T1 mapping application. Joseph Schoepf1 Curr Radiol Rep (2015) 3:32
4. Sheppard MN, Cane P, Florio R, et al. A detailed pathologic examination of heart tissue from three older patients with Anderson-Fabry disease on enzyme replacement therapy. Cardiovasc Pathol. 2010;19:293-301.
5. Sado DM, Flett AS, Banypersad SM, et al. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2010;98:1436-1441.
6. Elleder M, Bradova V, Smid F, et al. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry’s disease. Report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch A Pathol Anat Histopathol. 1990;417(5):449-55.