2749
Reperfusion Hemorrhage Following Prolonged Myocardial Ischemia Leads to Fatty Degeneration of Myocardial Infarctions via Iron-Mediated, Self-Perpetuating Loop of Foam Cell and Ceroid Accumulation1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Louisiana State University
Synopsis
Fatty infiltration within chronic myocardial infarctions (MI) is a common finding. It is typically observed in the peri-infarct border zone of old scars and has been linked to adverse outcomes in the chronic post-MI setting. To date, the trigger for fat deposition within old MI is unknown. Recent reports showed that iron deposits from hemorrhagic MI drive the recruitment of new monocytes/macrophages into the infarcted territory throughout the chronic phase after MI. Since iron-laden macrophages (siderophages) are prone to transforming into foam cells, we hypothesized that fatty degeneration of hemorrhagic myocardial infarctions has its origin in iron-driven foam cell formation.
PURPOSE
Fat deposition in old myocardial infarctions (MI) is a common finding. It is typically observed in the peri-infarct border zone of old scars and has been linked to adverse outcomes in the chronic post-MI setting. The mechanism of this fatty degeneration has conventionally been attributed to the process of “lipomatous metaplasia”. Lipomatous metaplasia describes the replacement of scar collagen by metaplastic adipose tissue as part of the healing cascade after MI. To the best of our knowledge, no studies have yet been undertaken to elucidate the mechanisms underlying fatty infiltration within the MI scar.
A common feature of many disease processes associated with pathological fat accumulation is the abnormal deposition of erythrocyte-derived iron. Interactions between iron and lipid metabolism have been extensively investigated with respect to coronary atherosclerosis and hepatic steatosis. Upon phagocytosis of oxidized red blood cells (oxRBC), macrophages tend to transform into foam cells and produce autofluorescent pigment with the characteristics of ceroid. The process of foam cell formation following erythrophagocytosis involves exocytosis of iron. Phagocytosis of this secreted iron by new macrophages can lead to a self-perpetuating and amplifying cycle of foam cell and ceroid accumulation.
A growing body of evidence now shows that hemorrhagic myocardial infarctions lead to chronic iron deposition. Moreover, recent reports indicate that iron deposits from hemorrhagic MI drive the recruitment of new monocytes/macrophages into the infarcted territory throughout the chronic phase after MI. In this study, we hypothesized that fatty infiltration in hemorrhagic myocardial infarctions has its origin in perpetual oxRBC-iron recycling and foam cell formation.
METHODS
Canines (n=14) were studied according to the protocols approved by the institutional Animal Care and Use Committee. All canines were subjected to 3-hour occlusion of the left anterior descending artery, followed by reperfusion.
Cardiac MRI: Seven days after MI, all dogs underwent T2* and Late Gadolinium Enhancement MRI in a 3T clinical MRI system (MAGNETOM Verio, Erlangen, Siemens Healthcare). T2*-weighted (multiple gradient-echo, TR=12ms, 6 TEs = 2.0ms–9.5ms with ΔTE=1.5ms, flip angle=10° and BW=930Hz/pixel) and the Late Gadolinium Enhancement (IR-FLASH acquired 10-15 minutes following intravenous gadolinium infusion (Magnevist, Bayer Healthcare Pharmaceuticals Inc., Wayne, NJ), optimal TI to null remote myocardium, TR/TE=3.0/1.5 ms, flip angle=25°, BW=586 Hz/pixel) were acquired along the short-axis direction covering the entire left ventricle. Commonly used imaging parameters for all the scans were: resolution = 1.4 x 1.4 x 6 mm3. All qualitative image analyses were performed using the cvi42 (Circle Cardiovascular Imaging Inc.). Animals were recovered and followed for 6 months post-MI at which time they were sacrificed.
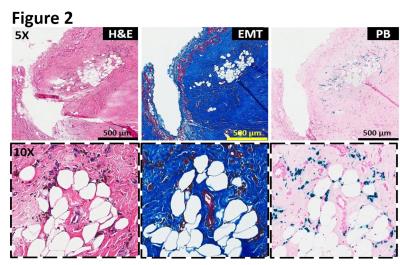
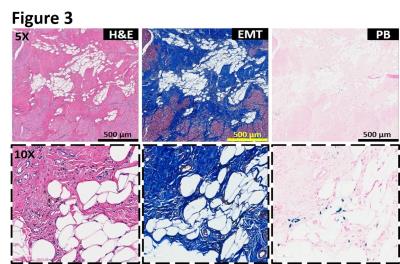
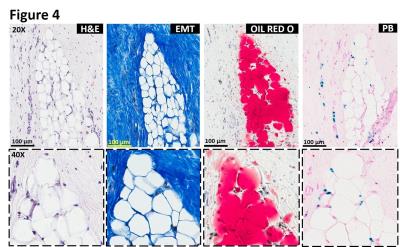
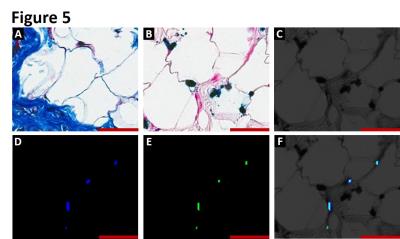
Histopathology: Explanted hearts underwent paraffin and frozen processing and histopathological evaluation. Both paraffin and frozen sections were stained with hematoxylin and eosin (H&E), elastin-modified Masson’s trichrome (EMT) and Prussian blue (PB) stains. Foam cell formation in the iron-laden border zone regions was confirmed by Oil Red O (ORO) staining on frozen sections. Sections stained with Prussian blue were examined for autofluorescence of ceroid under Leica SP5-X confocal microscope.
RESULTS
Intramyocardial hemorrhage was detected in 10 out of 14 dogs on day 7 post-MI by T2* MRI (Figure 1). Only dogs positive for intramyocardial hemorrhage in subacute MI showed evidence of fatty infiltration and persistent iron deposits in the border zones of chronic MI. Moreover, fat depots (foam cells) in the peri-infarct border zone regions exclusively co-localized with Prussian blue-stained iron (Figures 2, 3 and 4) and auto-fluorescent ceroid (Figure 5).
CONCLUSIONS
Herein we demonstrate that iron deposition from hemorrhagic myocardial infarctions is intimately linked with the process of fatty infiltration in the peri-infarct border zone of myocardial infarction scar. Moreover, our results indicate that iron deposits from hemorrhagic myocardial infarctions are being persistently recycled in a self-amplified loop of foam cell formation with perpetual production and deposition of intra-and extracellular ceroid. These findings thus form the first evidence that fat infiltration within myocardial infarction is driven by processes other than lipomatous metaplasia. We conclude that iron plays a critical role in adverse cardiac remodeling throughout the chronic phase of hemorrhagic myocardial infarctions.Acknowledgements
No acknowledgement found.References
No reference found.Figures
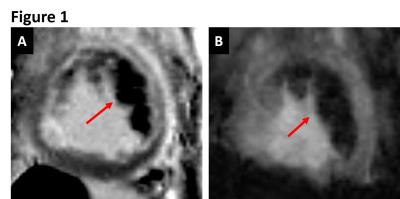
Figure 1. CARDIAC MRI: Representative LGE (A) and T2* (B) MRI acquired 7 days after acute MI confirming the presence of hemorrhage (arrows).