2745
Integrated Analysis of Cardiac Genetic and Structural Alterations in Left Ventricular Hypertrophy using the Supertoroidal Model1Harvard Medical School - Massachusetts General Hospital, Boston, MA, United States, 2Brigham and Woman’s Hospital, Harvard Medical School, Boston, MA, United States, 3Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, United States, 4University of São Paulo, São Paulo, Brazil
Synopsis
Response to disease occurs over many scales ranging from individual gene expression to whole organ physiology. We employed the supertoroidal model of the diffusion tensor to study the interaction between gene expression and microstructure of the heart. Left ventricular hypertrophy (LVH) was induced in C57Bl6 mice through aortic banding, and characterization of the cardiac microstructure was performed in vivo with DTI. The supertoroidal model was constrained by both diffusion information and gene expression data related to cardiomyocyte hypertrophy and myofiber orientation. Our model enabled further characterization of LVH by unifying information at different scales and across domains.
Purpose
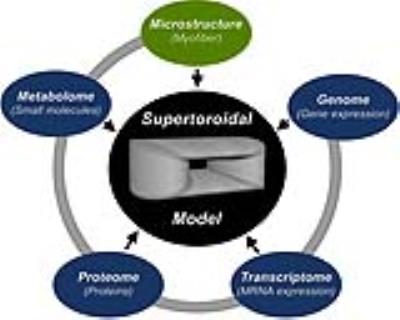
Response to disease occurs over many scales ranging from individual gene expression to whole organ physiology. Changes in the genome, transcriptome, proteome, metabolome, and ultimate structure of the heart have been described in a broad range of diseases.1,2 While of interest in isolation, an approach that integrates cardiac genetic and structural data would enable study of the interaction between these disparate domains in a variety of cardiac pathophysiology (Figure 1). Here, our unified analysis employs the multidimensional property of the supertoroidal model of the diffusion tensor3 to study the interaction between gene expression and microstructure of the heart.Methods
Left ventricular hypertrophy (LVH) was induced in C57Bl6 mice through banding of the transverse thoracic aorta for 4 weeks. In vivo diffusion tensor MRI (DTI) was performed in these aortic-banded mice (n=6) and in healthy, age-matched controls (n=6), on a 9.4T scanner (Bruker) equipped with a 1500 mT/m gradient insert.4 Two averages were acquired using a velocity-compensated pulsed gradient spin echo diffusion sequence at mid-systole with resolution of 156 µm3, b-value of 0 and 580 s/mm2, and 24 diffusion-encoding directions. Physiologic parameters were maintained to ensure normal left ventricular loading conditions. The supertoroidal model encodes the diffusion eigensystem described by the local dyadic diffusion tensor.5 Gene expression analysis of 31,556 genes was performed with the Affymetrix mouse array in the same aortic-banded mice and controls. Upregulated genes were classified based on their ability to influence cardiomyocyte hypertrophy or myofiber orientation, and were used to parameterize the shape (η1 and η2) of the supertoroid, respectively (Figure 2). An index of diffusivity derived from the toroidal model, the toroidal volume (TV),5 was then quantified with and without the genomic information.Results
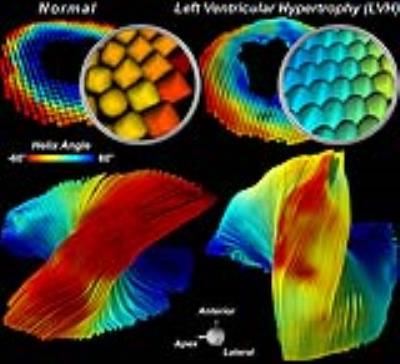
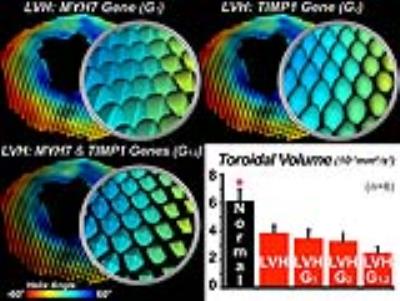
Supertoroids computed from the mice with LVH are more closely packed and have less sphericity (Figure 3). A rightward (positive) shift in the orientation of the supertoroids in the free wall of the LV was seen in the banded mice, and was also present in the tractograms. Aortic banding was associated with differential expression of 890 genes and a greater than 3-fold upregulation of 48 genes. Upregulated genes encoding for cardiomyocyte hypertrophy included myosin heavy chain 7 (MYH7). This was increased by 5-fold and was used to constrain η1 proportionally, further decreasing the sphericity of the supertoroids (Figure 4). Over 10 genes with the potential to influence the extracellular matrix and myofiber orientation were upregulated, including tissue inhibitor of metalloproteinase 1 (TIMP1), which was used to constrain η2, leading to a more ellipsoidal geometry. Constraining the supertoroids by both η1 and η2 markedly altered the shape of supertoroids, producing a composite representation of changes in gene expression and diffusivity. TV provided a quantitative measure of disease impact. Compared to normal myocardium,TV was reduced in LVH and showed significant further reductions when constrained by MYH7 and TIMP1.Discussion and Conclusion
Using an aortic-banded mouse model, we have shown how the interaction of multiple genetic factors influences the myofiber architecture. The supertoroidal model provides a basis for the integration of data from diverse domains, such as gene expression and tissue microstructure derived from DTI. This work is our first effort toward a multi-domain framework for the characterization and quantification of a disease process, in this case left ventricular hypertrophy. The supertoroidal model provides a mathematical relationship that captures the interaction between information at different scales and across domains, which can be evaluated both spatially and statistically, providing additional insight into the underlying pathology.Acknowledgements
No acknowledgement found.References
1. Mekkaoui C, Huang S, Chen HH, Dai G, et al. Fiber architecture in remodeled myocardium revealed with a quantitative diffusion CMR tractography framework and histological validation. J Cardiovasc Magn Reson. 2012;14:70.
2. Mekkaoui C, Reese TG, Jackowski MP, et al. Diffusion Tractography of the Entire Left Ventricle by Using Free-breathing Accelerated Simultaneous Multisection Imaging. Radiology. 2016:152613.
3. Mekkaoui C, Jackowski M, Martuzzi R, et al. Supertoroidal-based fusion of cardiac DT-MRI with molecular and physiological information. J Cardiovasc Magn Reson. 2010.
4. Sosnovik DE, Mekkaoui C, Huang S, et al. Microstructural impact of ischemia and bone marrow-derived cell therapy revealed with diffusion tensor magnetic resonance imaging tractography of the heart in vivo. Circulation. 2014;129:1731-41.
5. Mekkaoui C, Metellus P, Kostis WJ, et al. Diffusion Tensor Imaging in Patients with Glioblastoma Multiforme Using the Supertoroidal Model. PloS one. 2016;11:e0146693.
Figures