2738
CMR-Derived Regional T2, T1/ECV, Myocardial Velocities, and Dyssynchrony Influenced by Donor and Recipient Characteristics after Heart Transplantation1Radiology, Northwestern University, Chicago, IL, United States, 2Cardiology, Northwestern University, Chicago, IL, United States
Synopsis
Cardiac MRI is increasingly being used for cardiac allograft surveillance following transplantation, so it is important to investigate which recipient and donor characteristics influence several CMR parameters: global ventricular function, myocardial velocities, dyssynchrony, T2, native T1, and ECV. Notable associations with T2 included donor age, normalized recipient-donor age difference, and recipient weight. Peak diastolic longitudinal velocity was associated with donor age and cold ischemic time.
Introduction
Cardiac magnetic resonance (CMR) continues to gain acceptance in allograft surveillance following heart transplantation (Tx) due to its ability to measure global and regional left (LV) and right (RV) ventricular function and to quantify changes in regional myocardial tissue structure. CMR techniques include stacked LV volume measurement to assess global ejection fraction and regional strain using 2D cine SSFP MRI, tissue phase mapping (TPM) to measure regional myocardial velocities and dyssynchrony [1,2], T2-mapping to evaluate edema and inflammation [2-4], and pre- and post-contrast T1-mapping to calculate extracellular volume fraction (ECV) to assess fibrotic and interstitial changes [5,6]. Because Tx recipients and their donors are heterogeneous, it is important to examine which factors impact LV global and regional function, as well as myocardial tissue damage. The goal of this study was to apply comprehensive structure-function CMR to determine how age, sex, height, weight, BMI, donor ejection fraction, and cold ischemic time in a large cohort of Tx recipients influence several CMR parameters: global ventricular function, myocardial velocities and dyssynchrony, T2, native T1, and ECV.Methods
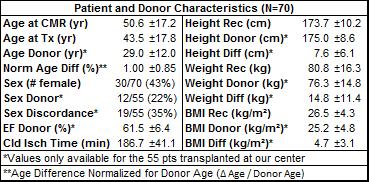
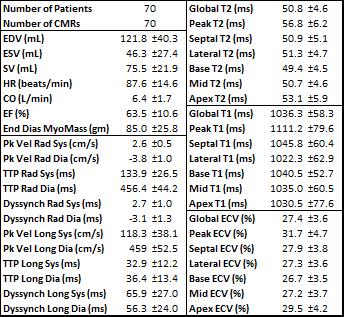
Cardiac MRI at 1.5T (Magnetom Aera or Avanto, Siemens, Erlangen, Germany) was performed in 70 Tx recipients prospectively recruited at a single major tertiary referral center from 2014-2016 (6.4±6.2 years post Tx, 23% within 1 year post Tx). All CMR examinations included 2D cine SSFP, T2-mapping, native and post-Gd T1-mapping, and TPM. Donor information was obtained from UNOS data available at the time of Tx (55 of the 70 pts). Global LV function parameters (EDV, ESV, SV, HR, CO, EF, end diastolic myocardial mass) were calculated from a short-axis stack of 2D cine SSFP images covering the entire LV. T2 and native and post-Gd T1 maps (to generate ECV fraction using hematocrit drawn at the time of CMR) in short-axis views of the LV at the base, mid, and apex were automatically reconstructed on the MRI system and further analyzed using dedicated software (cvi42, version 5.3.6, Circle, Calgary, Canada). TPM consisted of a black-blood cine 2D phase-contrast sequence that allowed for acquisition of tri-directional encoded LV velocities in a single breath hold (venc=25 cm/s, temporal resolution=24 ms, spatial resolution=2.0x2.0 mm², k-t GRAPPA acceleration with R=5). TPM data were acquired in LV basal, mid, and apical short-axis orientation and used to quantify myocardial peak and time to peak velocity (TTP) in systole and diastole using an in-house tool (Matlab, Mathworks, Natick, MA). The standard deviations of TTP across all 16 LV segments were used to evaluate the extent of radial and longitudinal dyssynchrony. A Pearson correlation analysis was performed between continuous recipient and donor characteristics and CMR parameters, and independent sample T-tests were performed of the CMR parameters for the categorical characteristics, all using SPSS (v23, IBM, Armonk, NY).Results
Recipient and donor characteristics are summarized in Table 1, and average CMR parameters in this cohort are reported in Table 2. Amongst the global LV function parameters, recipient and donor height were positively correlated with SV (r=0.37, p=0.006; r=0.29, p=0.033), and recipient height was positively correlated with CO (r=0.40, p=0.004). Donor-recipient age difference showed a negative correlation with SV (r=-0.41, p=0.010). Larger differences in weight and BMI were associated with lower EF (r=-0.30, p=0.030; r=-0.34, p=0.014). Correlation analysis results between age, height, weight, and BMI and T2 and TPM parameters are shown in Table 3. Global and regional T2 were positively associated with donor age (global r=0.36, p=0.033) and negatively associated with recipient-donor age difference (global r=-0.52, p=0.001), especially in lateral and basal regions. There were many associations between these characteristics and TPM parameters, notably between increasing diastolic radial and longitudinal TTP and donor weight (r=0.42, p=0.005; r=0.47, p=0.002). Systolic and diastolic radial function declined (decreased peak velocities, increased TTP and dyssynchrony) with increased recipient age at Tx (r=-0.33, p=0.014; r=0.28, p=0.041; r=0.29, p=0.031) and at the scan (r=-0.37, p=0.005; r=0.32, p=0.016; r=0.34, p=0.012). Increasing cold ischemic time was associated with decreased diastolic peak longitudinal velocities (r=-0.33, p=0.041). There were no strong associations between these characteristics and T1 or ECV parameters. T-tests showed decreased peak systolic longitudinal velocities in patients with recipient-donor sex discordance (p=0.002).Conclusion
Global LV function, T2, T1, ECV, and TPM parameters are each influenced by several recipient and donor characteristics. Further study of these factors is needed to reveal how they affect allograft health, to interpret CMR data accurately, and to optimize recipient-donor matching.Acknowledgements
Grant support by NHLBI R01 HL117888.References
1. Foll D, et al. Magnetic resonance tissue phase mapping of myocardial motion: New insight in age and gender. Circ Cardiovasc Imaging 2010; 3:54-64.
2. Markl M, et al. Myocardial T2-mapping and velocity mapping: Changes in regional left ventricular structure and function after heart transplantation. Magn Reson Med. 2012, published online.
3. Giri S, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009; 11:56.
4. Usman AA, et al. Cardiac magnetic resonance T2 mapping in the monitoring and followup of acute cardiac transplant rejection: A pilot study. Circ Cardiovasc Imaging 2012; 5:782-790.
5. Messroghli DR, et al. Modified looklocker inversion recovery (MOLLI) for high-resolution T1-mapping of the heart. Magn Reson Med. 2004; 52:141-146.
6. Lee JJ, et al. Myocardial T1 and extracellular volume fraction mapping at 3 Tesla. J Cardiovasc Magn Reson. 2011; 13:75.
Figures