2736
Multi-Slice GRE-MOLLI at 3T using Denoising with Low-Rank and Sparsity Constraints1Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States
Synopsis
Modified Look-Locker inversion recovery (MOLLI) uses bSSFP readout due to its high SNR, however, bSSFP is sensitive to off-resonance effects which result in banding artifacts. Recently, GRE has been proposed as an alternative readout for MOLLI, however, the low SNR efficiency of GRE-MOLLI is still a major problem. In this work, we propose to use a denoising reconstruction framework with low-rank and sparsity constraints to improve the low SNR of GRE-MOLLI. The proposed denoising method improved the low SNR of GRE-MOLLI, and multi-slice GRE-MOLLI is feasible for artifact-free T1 mapping with wider spatial coverage at high magnetic fields ($$$\geq 3T$$$).
Introduction
Modified Look-Locker inversion recovery (MOLLI)1 is a technique widely used for myocardial T1 mapping. The technique uses balanced steady-state free precession (bSSFP) readout due to its high signal-to-noise ratio (SNR) efficiency2. However, bSSFP is sensitive to off-resonance effects which often result in banding artifacts. Recently, spoiled-gradient echo (GRE) with low-flip angle excitations has been proposed as an alternative readout for MOLLI3,4,5 and its variants6 to achieve T1 maps with robustness to off-resonance effects. However, the low SNR efficiency of GRE-MOLLI is still a major problem that limits its practical usage. In this work, we propose to use a denoising reconstruction framework with low-rank and sparsity constraints to improve the low SNR of GRE-MOLLI. The performance of multi-slice GRE-MOLLI is verified via simulation and phantom study, and the feasibility of whole-heart T1 mapping using GRE-MOLLI is demonstrated via an in vivo study with a porcine model.Methods
Low-rank7 and sparsity constraints8 have been exploited for various applications including diffusion-weighted9,10 and accelerated cardiac imaging11. In this work, we have adapted the denoising reconstruction framework with low-rank and sparsity constraints to GRE-MOLLI case by formulating the problem as:
$$\hat{\rho}=arg\min_{\rho\in C^{N\times M}}\parallel d -\rho \parallel_2^2+\lambda\parallel\Psi(\rho)\parallel_1 s.t. rank(\rho)\leq L$$
where $$$d$$$ denotes measurement, $$$\rho$$$ denotes true noise-free GRE-MOLLI data, $$$\lambda$$$ denotes lagrangian multiplier, $$$\Psi$$$ denotes sparsifying transform, $$$C$$$ denotes the field of complex numbers, $$$N$$$ denotes number of voxels, and $$$M$$$ denotes number of time measurements. Low-rank constraint was used with the expectation that high spatio-temporal correlation exists between the noise-free, time-evolving voxel signals of GRE-MOLLI assuming homogenous T1 within the myocardium and blood pool. Total variation12 was used for to exploit the spatial correlation of myocardium and blood pool structure in the heart for effective edge-preserving smoothing. The above problem was solved similarly as in Ref.[11].
All experiments were performed on a PET-MR scanner (mMR Biograph, Siemens Healthcare, Erlangen, Germany) using body coil for transmission and body/spine matrix coils for signal reception. Simulations were performed using a steady-state spoiled GRE signal model13 to determine the Ernst angle of GRE readout for a range of T1 values and compare the signal GRE with bSSFP prior to experiments. The performance of GRE-MOLLI sequence was tested using a T1 phantom consisting of different concentrations of gadolinium (Gd) with simulated heart rate of 60beats/min prior to in vivo experiment. A porcine model was imaged under a study protocol approved by the IACUC. Cardiac images of an anesthetized porcine model were obtained at mid-ventricular short axis view with free-breathing using ECG-triggered single-shot acquisitions. Acquisitions were performed over 220ms window in end-diastole period of each cardiac cycle to minimize artifacts from cardiac motion.
2D multi-slice acquisitions were performed using MOLLI1 with 5-(3)-5-(3) scheme (i.e. 5 acquisition per IR set and 3 additional cardiac cycles to enable full recovery of longitudinal magnetization) over 16 heartbeats for each slice, and inversion times (TI) of 100 and 200ms were used for each IR set. The general imaging parameters were: field-of-view (FOV) = 360$$$\times$$$304mm2, matrix size = 192$$$\times$$$162, number of slices=16, and slice thickness=6 mm. GRAPPA14 and POCS15 reconstructions were performed prior to data analysis. T1 was estimated for each voxel via least-square fitting to three-parameter IR signal model3 using variable projection algorithm16 and conventional LL equation for T1 estimation17. Relative bias of T1 from GRE-MOLLI was calculated using T1 from bSSFP-MOLLI as reference3.
Results
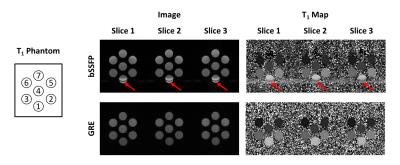
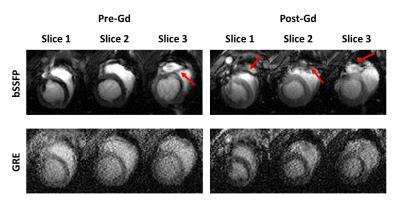
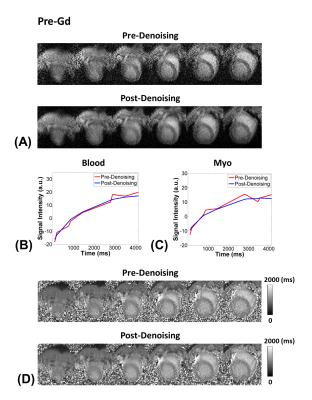
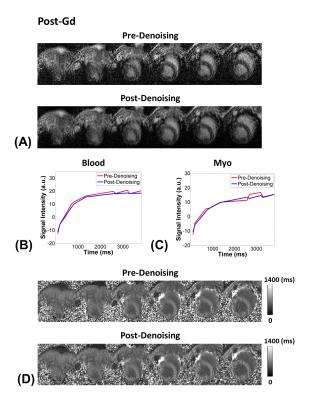
Considering the T1 of myocardium and blood at 3T, flip angles of 4-7$$$\circ$$$ is recommended for myocardial T1 mapping with GRE-MOLLI (Figs. 1A and B). The phantom results showed that multi-slice T1 mapping is feasible using GRE-MOLLI, with T1 values showing relative bias of 5.0 $$$\pm$$$ 0.6 % compared to those from bSSFP-MOLLI (Fig. 2). The bSSFP readout theoretically (Fig. 1C) and experimentally (Figs. 2 and 3) showed higher SNR than GRE, however, usage of bSSFP readout for MOLLI resulted in banding artifacts in regions with off-resonance (red arrows in Figs. 2 and 3). Usage of GRE readout for MOLLI did not result in any apparent artifacts (Figs. 2 and 3), indicating potentially usefulness of GRE over bSSFP for T1 mapping with wider spatial coverage at clinical or higher magnetic field strengths. The proposed denoising method clearly improved the SNR of GRE-MOLLI spatially (Figs. 4A and 5A) and temporally (Figs. 4B,C and 5B,C) and the T1 maps (Figs. 4D and 5D).Conclusion
The proposed denoising method with low-rank and sparsity constraints improved the low SNR of GRE-MOLLI. Multi-slice GRE-MOLLI is feasible for artifact-free T1 mapping with wider spatial coverage at 3T.Acknowledgements
No acknowledgement found.References
1. Messroghli, Daniel R., et al. "Modified Look-Locker Inversion recovery (MOLLI) for high-resolution T1 mapping of the heart." Magnetic Resonance in Medicine 52.1 (2004): 141-146.
2. Scheffler, Klaus et al. "Principles and applications of balanced SSFP techniques." European Radiology 13.11 (2003): 2409-2418.
3. Cameron, Donnie et al. "Selection of magnetization catalyzation and readout methods for modified Look–Locker inversion recovery: AT 1 mapping primer." Magnetic Resonance Imaging 33.4 (2015): 363-373.
4. Shao, Jiaxin et al. "Myocardial T1 mapping at 3.0 tesla using an inversion recovery spoiled gradient echo readout and bloch equation simulation with slice profile correction (BLESSPC) T1 estimation algorithm." Journal of Magnetic Resonance Imaging 43.2 (2016): 414-425.
5. Rodgers, Christopher T. et al. "Inversion recovery at 7 T in the human myocardium: measurement of T1, inversion efficiency and B1+." Magnetic Resonance in Medicine 70.4 (2013): 1038-1046.
6. Jang, Jihye, et al. "Comparison of spoiled gradient echo and steady-state free-precession imaging for native myocardial T1 mapping using the slice-interleaved T1 mapping (STONE) sequence." NMR in Biomedicine 29.10 (2016): 1486-1496.
7. Liang, Zhi-Pei. "Spatiotemporal imaging with partially separable functions." 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro. IEEE, 2007.
8. Haldar, Justin P., et al. "Spatiotemporal imaging with partially separable functions: A matrix recovery approach." 2010 IEEE International Symposium on Biomedical Imaging: From Nano to Macro. IEEE, 2010.
9. Haldar, Justin P., et al. "Improved diffusion imaging through SNR-enhancing joint reconstruction." Magnetic resonance in medicine 69.1 (2013): 277-289.
10. Lam, Fan, et al. "Denoising diffusion-weighted magnitude MR images using rank and edge constraints." Magnetic resonance in medicine 71.3 (2014): 1272-1284.
11. Zhao, Bo, et al. "Image reconstruction from highly undersampled-space data with joint partial separability and sparsity constraints." IEEE transactions on medical imaging 31.9 (2012): 1809-1820.
12. Chambolle, Antonin. "An algorithm for total variation minimization and applications." Journal of Mathematical imaging and vision 20.1-2 (2004): 89-97.
13. Haacke, E. Mark, et al. Magnetic resonance imaging: physical principles and sequence design. Vol. 82. New York:: Wiley-Liss, 1999.
14. Griswold, Mark A. et al. "Generalized autocalibrating partially parallel acquisitions (GRAPPA)." Magnetic Resonance in Medicine 47.6 (2002): 1202-1210.
15. Cuppen, Jan et al. "Reducing MR imaging time by one-sided reconstruction." Magnetic Resonance Imaging 5.6 (1987): 526-527.
16. Golub, Gene et al. "Separable nonlinear least squares: the variable projection method and its applications." Inverse Problems 19.2 (2003): R1.
17. Deichmann, R. et al. "Quantification of T 1 values by SNAPSHOT-FLASH NMR imaging." Journal of Magnetic Resonance 96.3 (1992): 608-612.
18. Lee, Jason J., et al. "Myocardial T1 and extracellular volume fraction mapping at 3 tesla." Journal of cardiovascular magnetic resonance 13.1 (2011): 1.
Figures