2734
Quasi black blood T1-mapping using slice-selective TRASSI for improved visualization of the myocardium1Department of Internal Medicine I - Cardiology, University Hospital Würzburg, Würzburg, Germany, 2Comprehensive Heart Failure Center (CHFC), University Hospital Würzburg, Würzburg, Germany, 3Experimental Physics 5, University of Würzburg, Germany, 4Magnetic Resonance and X-ray Imaging MRB, Development Center X-ray Technology EZRT Fraunhofer Institute for Integrated Circuits IIS, Würzburg, Germany
Synopsis
Currently available T1 mapping techniques have only restricted capabilities for the visualization of the right myocardium and they have the limitation that after contrast agent application the T1-contrast between blood pool and fibrotic myocardium is partially very low.
The quasi black blood TRASSI sequence shows improved abilities for the visualization and T1-quantification of myocardial structures. So it might be suited in clinical routine for a clear visualization of the right myocardium or for the detection of slight endocardial infarctions, because it is an ultra-fast and robust cardiac T1-mapping method with a total acquisition time of less than 7s.
Introduction
Late gadolinium enhanced (LGE) cardiovascular magnetic resonance (CMR) is the clinical gold-standard for the visualization of myocardial infarction or fibrosis in non-ischemic cardiomyopathies1. However, LGE CMR provides only restricted possibilities for the identification of slight abnormalities of the myocardium. Hence, cardiac T1-mapping has become an increasingly important imaging technique over the last years, which establishes new non-invasive diagnostic possibilities. Several recent studies have shown that cardiac T1 measurements can be used to acquire diverse morphological and functional information2-4. Potential capabilities include distinguishing between healthy and diseased cardiac tissue. However, currently available techniques have the limitation that after contrast agent application the T1 time difference between blood pool and fibrotic myocardium is partially very low prohibiting a clear detection and investigation of the fibrotic area. Furthermore, common T1-mapping methods have only restricted capabilities for visualization of the right myocardium.
In the current work a very fast T1-mapping sequence was implemented using a slice selective inversion pulse for an improved visualization of the right myocardium and a better differentiation between blood pool and fibrotic tissue.
Methods
All measurements were performed on a 3.0 T whole-body imaging system. For T1-mapping a triggered radial single-shot inversion recovery sequence (TRASSI) was used3,4. At the beginning of the TRASSI sequence a slice-selective 180° inversion pulse is given followed by multiple radial imaging blocks acquired with a golden-ratio-based5 trajectory profile. Images were reconstructed using a modified KWIC-filter6 to generate an image-series with up to 140 Snap-Shot-FLASH images with different inversion times (TIs). For data analysis a custom fitting algorithm was used that simulates the pulse sequence with the known timings.
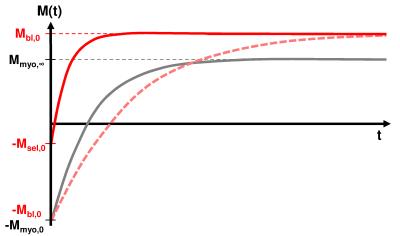
Using a slice-selective inversion pulse the magnetization of the blood pool shows an extraordinary behavior (Figure 1) due to the inflow of non-inverted fresh blood7. This results in a very low fitted T1-time for the blood pool appearing dark or “quasi black” in the generated T1-maps. Due to the strong signal difference between blood and myocardium the blood pool can be excluded from the slice-selective inverted T1-map using a threshold masking tool at one of the reconstructed Snap-Shot-FLASH images (with the highest contrast between blood and myocardium) receiving a masked quasi black blood T1-map.
Four healthy volunteers and 1 patient with acute myocardial infarction were investigated with the implemented slice-selective TRASSI pulse sequence. The data acquisition was performed in end-expiration breath holds each acquisition with a duration of less than 7s. Sequence parameters were: FOV = 300 x 300 mm², TR = 4.06 ms, TE = 2.00 ms, FA = 7°, slice thickness = 8 mm, reconstructed in-plane resolution = 1.2 mm.
Results
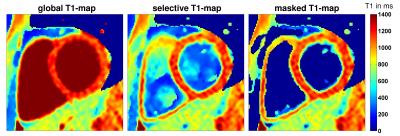
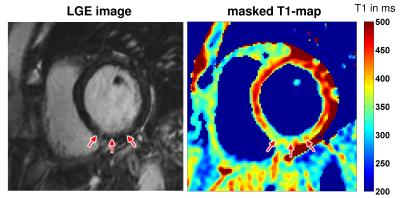
In Figure 2 exemplary T1-maps generated with global-selective respectively slice-selective inversion pulses and a masked quasi black blood T1-map from a healthy volunteer are depicted. In the masked and the slice-selective T1-maps the right myocardium can be visualized very well whereas in the global T1-map it cannot be identified. Figure 3 shows a LGE image and a masked slice-selective T1-map from a patient with acute myocardial infarction. Here, the LGE image enables no clear distinction between healthy myocardium, scar, and blood pool. In contrast, the masked T1-map enables a clear differentiation between blood pool, scar, and healthy myocardium.Discussion and conclusion
The presented small preliminary study shows the improved capabilities of our slice-selective TRASSI sequence for the visualization and T1-quantification of myocardial structures. This includes the visualization of the right myocardium that could be clearly identified with sharp edges in high spatial resolution. Furthermore, the technique allows a clear differentiation between remote myocardium, scar, and blood pool in patients with myocardial infarction. The presented method allows a very fast myocardial T1 quantification generating accurate T1-maps within a total acquisition time of less than 7s.
Hence, the slice-selective TRASSI sequence might potentially be suited to be used as the new gold standard in clinical routine for the visualization of the right myocardium or for slight endocardial infarctions, because it is an ultra-fast and robust cardiac T1-mapping method.
Acknowledgements
This work was supported by the Federal Ministry for Education and Research of the Federal Republic of Germany (BMBF 01EO1504, MO6 to Peter Nordbeck and Kristina Lorenz).References
1. R.J. Kim, D.S. Fieno, T.B. Parrish, K. Harris, E.L. Chen, O. Simonetti, J. Bundy, J.P. Finn, F.J. Klocke, R.M. Judd. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999 Nov 9;100(19):1992-2002.
2. D. Gensler, P. Mörchel, F. Fidler, O. Ritter, H.H. Quick, M.E. Ladd, W.R. Bauer, G. Ertl, P.M. Jakob, P. Nordbeck. Myocardial T1: quantification by using an ECG-triggered radial single-shot inversion-recovery MR imaging sequence. Radiology. 2015 Mar;274(3):879-87.
3. D. Gensler, P. Mörchel, F. Fidler, O. Ritter, H.H. Quick, M.E. Ladd, W.R. Bauer, P.M. Jakob, P. Nordbeck. Fast cardiac T1 quantification with an ECG-triggered radial single-shot inversion recovery sequence (TRASSI). Annual Meeting ISMRM, Salt Lake City 2013, P1366, Poster.
4. D.R. Messroghli, A. Radjenovic, S. Kozerke, D.M. Higgins, M.U. Sivananthan, J.P. Ridgway. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004 Jul;52(1):141-6.
5. S. Winkelmann, T. Schaeffter, T. Koehler, H. Eggers, O. Doessel. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007 Jan;26(1):68-76.
6. H.K. Song, L. Dougherty. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med. 2000 Dec;44(6):825-32.
7. T. Kampf, X. Helluy, F.T. Gutjahr, P. Winter, C.B. Meyer, P.M. Jakob, W.R. Bauer, C.H. Ziener. Myocardial perfusion quantification using the T1 -based FAIR-ASL method: the influence of heart anatomy, cardiopulmonary blood flow and look-locker readout. Magn Reson Med. 2014 May;71(5):1784-97.
Figures