2731
Improved Myocardial T1 mapping using a Novel Motion-Insensitive Reconstruction1Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Myocardial T1 mapping sequences are commonly performed under breath-hold conditions. However, motion between T1-weighted images can be observed in ~50% of acquisitions. Image registration algorithms can be used for motion correction but are not available on all scanners, can occasionally fail and remain challenging using low contrast sequences such as saturation-based T1 mapping techniques. In this study we sought to develop and evaluate a novel motion-insensitive T1 mapping reconstruction approach which automatically discards misaligned/artefact T1-weighted images.
Purpose
Quantitative myocardial T1 mapping is a promising technique for non-invasive assessment of cardiomyopathies1. Although myocardial T1 mapping sequences are commonly performed under breath-hold conditions2, motion can be observed between T1-weighted images in ~50% of acquisitions3,4. Image registration algorithms can be used for motion correction3,4 but are not available on all scanners, can occasionally fail and remain challenging using low contrast sequences such as saturation-based T1 mapping techniques. In this study we sought to develop and evaluate a novel motion-insensitive T1 mapping reconstruction approach which automatically discards misaligned/artefact T1-weighted images.Methods
Proposed reconstruction: T1-weighted image selection is achieved using an iterative process. At each iteration, several T1 map are reconstructed using different image subsets (all images excluding one). T1 fitting is performed using an exhaustive search of the minimal error between the normalized measured signal and a dictionary of a normalized 1-parameter model (S(t)=1–2*e(-t/T1)). The mean fitting error of each reconstruction (ME) is computed over the myocardium/blood area (obtained by manual delineation of the epicardial contour on the first image, which serves as reference). An image is discarded when its associated subset (where this image was excluded) leads to substantial ME reduction (ME < Median - 6*median absolute deviation (MAD) of all MEs). This process is then iterated if an image has been discarded. A maximum number of iteration of 3 was used. Final T1 maps are reconstructed using the conventional approach (i.e. 3-parameter model fit with Look-Locker correction5) and the preselected T1-weighted images. This iterative reconstruction process has been implemented on graphic processing unit (GPU) to reduce the computation time.
Experimental validation: All imaging was performed using a 1.5T Magnetom Aera scanner (Siemens Healthcare, Erlangen, Germany). All T1 mapping scans used a MOLLI sequence with bSSFP readout (TR/TE=2.5ms/1.2ms, flip angle=35°, FOV=360×306mm2, voxel size=2.2×2.5mm2, slice thickness=8mm, bandwidth=1085Hz/Px, GRAPPA acceleration factor=2, partial Fourier=0.87, MOLLI scheme: 5-(3)-3). All reconstructions were performed offline using an in-house software.
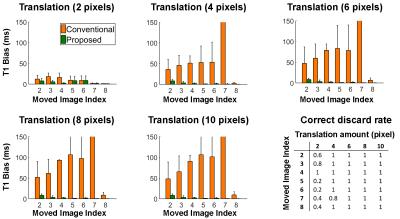
Study #1: Evaluation of the algorithm using in-vivo data with simulated motion. Five healthy volunteers were recruited and imaged using the MOLLI sequence acquired under breath-hold conditions (note the scan was repeated if the breath-hold was not successful). Multiple datasets were then simulated from each volunteer by introducing a 1D foot-head translation motion (amplitude={2,4,6,8,10} pixels) in one T1-weighted image (thereafter referred to as moved image). Each dataset was reconstructed using the proposed and the conventional (i.e. using all images) approaches. Reference MOLLI T1 maps were also reconstructed from each volunteer using the raw (no motion) MOLLI images and the conventional 3-parameter model fitting. Correct discard rate and T1 bias over the entire myocardium (with respect to reference MOLLI T1 map) were evaluated.
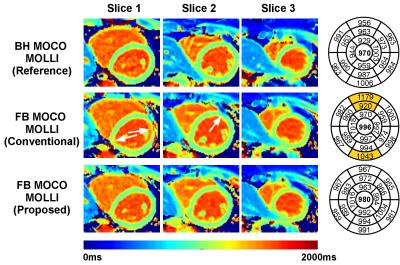
Study #2: Demonstration of proposed approach in the presence of physiological motion. One healthy volunteer was recruited and imaged twice using the MOLLI sequence: once under breath-hold conditions, one under free-breathing conditions. Each scan was reconstructed using the proposed and the conventional MOLLI reconstruction.
Results
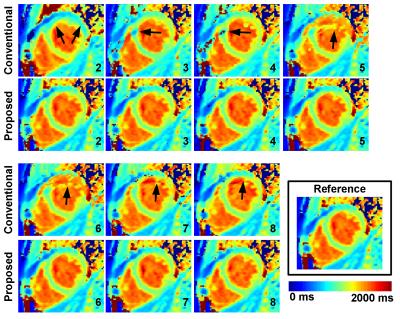
Study #1. One misaligned image in the MOLLI series can lead to important average myocardial T1 bias using the conventional reconstruction (>100ms, see Figure 1). The proposed approach could identify the motion-corrupted images in the majority of simulated cases and reduced T1 bias to <10ms in all tested cases. Figure 2 shows representative T1 maps reconstructed from different simulated motion cases from the same dataset using the proposed and conventional approaches. The proposed reconstruction improved the quality of native T1 maps in all cases and led to similar quality as the reference T1 map.
Study 2. The image registration step failed in the free breathing MOLLI acquisition and resulted in T1 bias in few myocardial segments using the conventional reconstruction (highlighted segments in bullseye plot in Figure 3). The proposed reconstruction provided improved T1 map quality similar to the breath-hold MOLLI acquisition.
The computation time of the proposed image selection process was 500ms in the worst case (3 iterations).
Discussion
The proposed reconstruction was found
superior to the conventional T1 mapping reconstruction technique in the
presence of uncorrected motion between T1-weighted images. In the absence of
motion, this technique would simply perform the conventional reconstruction. This
technique currently requires the manual delineation of the epicardial LV border
in one image. Future work will include the integration of an automatic
segmentation of the LV to provide a fully automatic reconstruction. Conclusions
The proposed reconstruction approach enables identification of misaligned images and reduces motion-related T1 reconstruction artefacts. The proposed GPU implementation enables clinical feasibility of the technique.Acknowledgements
This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London, and by the NIHR Healthcare Technology Co-operative for Cardiovascular Disease at Guy’s and St Thomas’ NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of HealthReferences
[1] Moon JC, et al., Myocardial T1 mapping and extracellular volume quantification. J Cardiovasc Magn Reson 2013;15(1):92
[2] Messroghli DR, et al., Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52(1):141–146
[3] Xue H, et al., Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med, 2012;67(6):1644-1655
[4] Roujol S, et al., Adaptive registration of varying contrast-weighted images for improved tissue characterization (ARCTIC): Application to T1 mapping. Magn Reson Med, 2015; 73(4):1469-1482
[5] Deichmann R, Haase A. Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson, 1992;96(3):608–612.
Figures