2724
End-systolic myocardial T1 mapping using a spoiled steady-state approach: Towards reducing the confounding effect of intra-myocardial blood on native T11Biomedical Imaging Research Institute, Dept. of Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Recent results in myocardial tissue characterization using cardiac MRI have shown that native myocardial T1 values are confounded by intra-myocardial water content, mainly driven by
Purpose
It has been recently established that a significant contributor to myocardial native T1 values is intra-myocardial water content, mainly driven by intra-myocardial blood volume (MBV).1,2 In fact, MBV can vary due to physiologic effects and may act as a major confounder for the quantified native T1 values. Therefore, assessment of native T1 free of the confounding effect of blood/water content can potentially improve the ability of native T1 as a reliable imaging marker for detecting myocardial pathologies, specifically diffuse myocardial fibrosis. In this work, we developed and tested a free-breathing native T1 mapping method that, in contrast to MOLLI-based methods, avoids any magnetization preparation and therefore can achieve much higher “temporal resolution” – enabling it to capture purely-systolic or purely-diastolic T1 maps. Since MBV is minimal during the end-systolic phase, such an approach has the potential to generate native T1 maps with minimal confounding effect from MBV.Methods
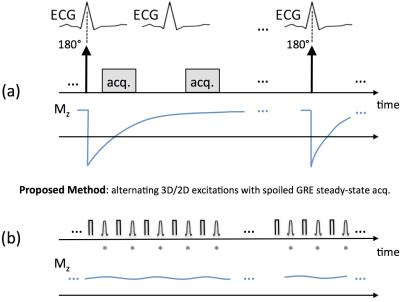
Figure 1 describes the proposed acquisition scheme, which continuously acquires data using a spoiled steady-state approach with alternating 3D/2D excitation and without breath-holding. The acquisition was performed twice with two different flip angles (FAs) with a fixed TR = 5 ms (FAs = 10° and 3°; scan time for each FA: 45 seconds) to be used for pixel-wise T1 mapping based on the DESPOT1 framework.3
Because of the magnetization preparation (“mag. prep.”), the imaging data in MOLLI-based methods inherently reflects the native T1 value averaged throughout the cardiac cycle over multiple heartbeats, as schematically depicted by the Mz evolution curve in Fig. 1(a). In contrast, the proposed method, shown in Fig. 1(b), does not use a mag. prep. and instead acquires the data in steady state (spoiled gradient-echo), hence enabling native T1 mapping of a specific cardiac phase (e.g., end systole) once the data is retrospectively “self-gated” to that particular cardiac phase. The proposed pulse sequence ignores the ECG signal and uses an alternating 3D/2D excitation scheme to drive the magnetization to steady state, despite the periodic through-plane motion and in-flow in the myocardial region. The 2D readouts (marked with a star in Fig. 1) are acquired continuously using a golden-angle radial trajectory and the 3D readouts were used for cardiac and respiratory self-gating.
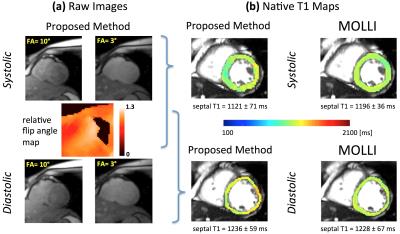
FA mapping was performed using an improved version of a previous method to account for B1+ inhomogeneity (12-second scan).4 The image reconstruction method employed a self-gating approach using the acquired 3D readouts with radial SENSE reconstruction of the image using the 2D readouts (in-plane resolution: 1.8 x 1.8 mm). The reconstructed images (end-systolic and mid-diastolic) and the relative FA map were used to generate a pixel-wise native T1 map using the DESPOT1 method.3
Healthy volunteers (n=9; age: 31±7) were studied on a 3T clinical scanner using the proposed method and the vendor-provided MOLLI 5(3)3 sequence with inline motion correction5 for native T1 mapping of the mid ventricular slice in systole and diastole.
Results
Figure 2 provides representative results for the proposed method including raw images for the two FAs and the generated FA map in (a), and the resulting native T1 maps (systolic and diastolic) in (b) with comparison to MOLLI. In the studied subjects, there was a significant difference between the end-systolic and mid-diastolic mean T1 values for the proposed method (1175 ms vs. 1273 ms, p-value < 0.05). The average of systolic and diastolic T1 values in each subject was similar for MOLLI versus the proposed method (MOLLI: 1203 ms, proposed method: 1219 ms; p-value = not significant).Discussion & Conclusions
Eliminating the confounding factors in myocardial T1 mapping is a major challenge in myocardial tissue characterization using cardiac MRI. We have developed and tested a new approach for free-breathing native T1 mapping that avoids magnetization preparation. This key feature effectively reduces the confounding effect of intra-myocardial blood volume (MBV) on native T1 values by limiting the "T1-fitting information" to the end-systolic phase (which has the lowest MBV) and avoiding contamination of the data by the diastolic phases (which have high MBV). The significantly lower native T1 values quantified using the proposed method in the end-systolic phase vs. mid-diastolic phase (Fig. 2) are consistent with this phenomenon. Overall, the promising results — specifically, high image quality and close agreement with MOLLI — point to the potential of this approach for highly accurate end-systolic free-breathing T1 mapping.Acknowledgements
No acknowledgement found.References
1. Liu A, Wijesurendra RS, Francis, JM, et al. Adenosine stress and rest T1 mapping can differentiate between ischemic, infarcted, remote, and normal myocardium without the need for gadolinium contrast agents. J Am Coll Cardiol (JACC): Cardiovascular Imaging 2016;9(1):27-36.
2. Mahmod M, Piechnik SK, Levelt E, et al. Adenosine stress native T1 mapping in severe aortic stenosis: evidence for a role of the intravascular compartment on myocardial T1 values. J Cardiovasc Magn Reson 2014;16:92.
3. Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med 2003;49(3):515-26.
4. Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn Reson Med 2010;64(2):439-46.
5. Xue H, Greiser A, Zuehlsdorff S, et al. Phase-sensitive inversion recovery for myocardial T1 mapping with motion correction and parametric fitting. Magn Reson Med 2013;69(5):1408-20.
Figures