2693
A chronic in situ coil system adapted for intracerebral stimulation during MRI in rats1Psychiatry, McGill University, Montreal, QC, Canada, 2Biomedical Engineering, McGill University, Montreal, QC, Canada, 3Psychology, Northeastern University, Boston, MA, United States
Synopsis
We describe the fabrication and performance of a chronic in situ coil system designed to allow focal brain stimulation in awake rats while acquiring highly resolved MRI data. We developed a subcutaneously implantable receive-only surface radiofrequency coil to be fitted immediately adjacent to the rat skull surface during the cannulation procedure. SNR performance of the coil was superior to three commercially-available coils, in some instances by a factor of three. Widespread BOLD was observed in response to bicuculline and morphine microinfusions. This approach enables mapping the functional response to highly targeted stimuli such as microinfusions or optogenetics.
Purpose
Recent MR imaging studies in small animals have demonstrated the possibility to combine functional imaging with intracranial procedures, such as optogenetics1, deep brain stimulation2, or microinfusions3. Such intracranial manipulations offer the potential to stimulate specific populations of neurons, either anatomically or by cell type, thus enabling imaging studies of whole brain functional changes associated with highly targeted stimuli. One obstacle to performing MRI in animals with intracranial implants is the availability of suitable RF coils. Many commercially available coils do not allow for through passage of wiring/tubing for intracranial procedures, thus the main purpose of this study is describe the fabrication and performance of a chronic in situ coil system designed to allow focal brain stimulation in awake rats while acquiring highly resolved MRI data.Methods
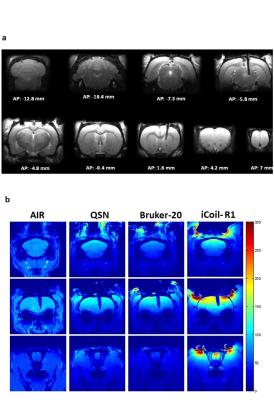
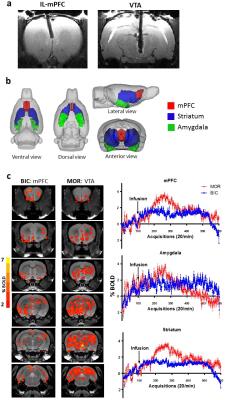
The RF coil was designed to be fitted subcutaneously, proximal to the skull, during the cannulation surgery (Fig. 1), while achieving comparable or improved SNR performance of commercially available surface coils optimized for rat brain imaging. Hence, the coil was tested against three commercially-available coils: a 45mm volumetric coil optimized for rat imaging, a rat brain optimized receive (Rx) only quadrature surface coil, and an Rx-only 20mm open loop coil. To allow removal of the coil circuitry when the animal is not being scanned, a detachable interfacing mechanism between the PCB and coil loop was developed. Two rats were fitted with both the subcutaneous implantable coil and additional intracranial cannula implants for microinfusions, while one rat was fitted with an intracranial cannula implant only for imaging with commercially available coils described above. Four rats were fitted with the implantable coil only, in order to assess possible inter-subject variability in SNR. All scans were performed on a horizontal bore Bruker 7T (70/30 USR; Biospec) preclinical animal MRI system upgraded with an actively shielded 120mm inner diameter gradient insert (650mT/m in 150μs) at the Douglas Brain Imaging Centre in Montreal. An EPI-based functional MRI sequence (TR/TE = 1000/15 ms, Segments = 3, Repetitions = 600, Effective spectral bandwidth = 300,000Hz, Slices = 20, Slice thickness = 1.2 mm, Spatial resolution = 0.3 mm x 0.3 mm, Matrix size = 96 x 96) was used to assess BOLD signal changes following intracranial infusion (0.5 µl) of either Bicuculline (20 ng/µl) in the infralimbic prefrontal cortex, or Morphine (20 µg/µl) in the ventral tegmental area (VTA). Functional images were analyzed using Medical Image Visualization and Analysis (MIVA; Ekam Solutions, Boston, MA, USA).Results
The rat head optimized AIR coil shows comparable or improved SNR coverage in the basal regions relative to the three Rx-only surface coils (Fig. 2). As expected, all of the surface coils tested produced higher SNR in the dorsal brain regions proximal to the coil. The implanted coil (iCoil) SNR performance was superior to the commercially available coils, with the exception of the ventral region of the cerebellar area compared to the AIR coil. The iCoil demonstrates superior SNR coverage (anterior-posterior direction) relative to all coils. Functional analysis revealed significant increases in average BOLD signal intensity within in several brain regions, including (but not limited to) striatum, mPFC, amygdala and motor cortex in response to both Biccuculine and Morphine infusions (Fig. 3). Specifically, intra-mPFC infusion of BIC shows a modest, albeit statistically significant increase (1-2%) in the average BOLD signal within the mPFC, striatum and amygdala. A more robust effect was recorded in response to the intra-VTA infusion of MOR, with the average BOLD signal increasing up to 3-4% in the mPFC, striatum.Conclusion
A new approach was demonstrated for high-SNR MR imaging of the brain in rats with intracranial implants using an implantable surface coil. This approach enables mapping the functional response to highly targeted stimuli such as microinfusions or optogenetics.
Acknowledgements
This work was supported by the Natural Sciences and Engineering Council of Canada (NSERC, # RGPIN-2014-07072, awarded to J.N.) and Fonds de recherche du Québec – Santé (FRQS, # 26679, awarded to N. R.)References
1. Abe Y, Sekino M, Terazono Y, Ohsaki H, Fukazawa Y, Sakai S, Yawo H, Hisatsune T. Opto-fMRI analysis for exploring the neuronal connectivity of the hippocampal formation in rats. Neuroscience research 2012;74(3-4):248-255.
2. Van Den Berge N, Vanhove C, Descamps B, Dauwe I, van Mierlo P, Vonck K, Keereman V, Raedt R, Boon P, Van Holen R. Functional MRI during Hippocampal Deep Brain Stimulation in the Healthy Rat Brain. PloS one 2015;10(7):e0133245.
3. Kim JH, Astary GW, Nobrega TL, Kantorovich S, Carney PR, Mareci TH, Sarntinoranont M.
Figures