2690
Sensitive Imaging of Vascular Walls with an Endo-esophageal Wireless Amplified NMR Detector (WAND)1Radiology, Provincial People's Hospital, Guiyang, People's Republic of China, 2Radiology, Michigan State University, East Lansing, MI, United States
Synopsis
To improve the detection sensitivity of MRI, a Wireless Amplified NMR Detector (WAND) is developed to image surrounding vessels from inside the esophagus. This cylindrical detector is a double frequency resonator with a single metal wire that is self-connected by a pair of varactors. It can convert wirelessly provided pumping power into amplified MR signals. When the detector is inserted inside the esophagus, vessel walls of the vertebrate artery and basal artery can be identified with greatly improved clarity. This detector will be useful to characterize subtle lesions in inflamed vessels.
Introduction
MRI can in principle be used to directly image and characterize atherosclerotic plaques. However, such applications are often hindered by its limited sensitivity, especially for regions deep lying inside the body. Recently, it has been demonstrated that the local detection sensitivity can be greatly improved with a Wireless Amplified NMR Detector (WAND) that can sensitively detect and simultaneously amplify MR signals from a close enough distance1. This detector can be inserted into the esophagus to image surrounding vessels at higher spatial resolution, enabling better identification of subtle ruptures in dissected walls.Methods
WAND can be implemented as a nonlinear double frequency resonator. This cylindrically-shaped detector (Fig. 1a) consists of three leg inductors (L1, L3, L2) that are serially connected. The gaps between legs and rings are bridged by two identical zero-biased varactors (C1 and C2). L1 and L2 are symmetrically distributed with respect to L3. Their span angle ψ is empirically adjusted near 90º to make the detector’s higher resonance frequency approximately twice its lower resonance frequency. The lower resonance mode receives MR signals at the Larmor frequency ω1, and the higher resonance mode receives a pumping signal at a frequency ω3 that is slightly above 2ω1. The frequency offset between ω3 and 2ω1 should be at least the imaging bandwidth, so that the “idler signals” created at the difference frequency ω2 = ω3 – ω1 can be filtered out to eliminate destructive interference with MR signals at ω1. Fig. 1b shows the front and rear views of a 9.2-mm long WAND. It is made of a 32 gauge copper wire mounted on a 3-mm diameter polyurethane cylinder. The metal wire is self-connected by two varactors shown in black (BB145B, NXP Semiconductors). The detector is then coated and inserted into the rat esophagus. The rat was anesthetized with isoflurane and secured in the prone position under ventilation. The insertion depth of the WAND is empirically adjusted by an insertion rod until the WAND’s sensitive region coincides with the regions of interest. A four-element surface coil for rat brain (Bruker Biospin) is used externally to receive amplified signals emitted from inside the body. A pair of pumping loops is placed orthogonally to the surface coil (Fig. 1c). The entire assembly was then inserted into a 7T magnet equipped with a 77-mm bore volume coil and an AVANCE III console (Bruker Biospin). Fast spin-echo sequence is subsequently used to image vessel walls in the presence of blood suppression.Results
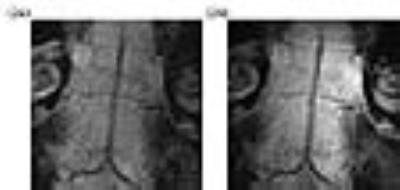
Figs. 2a and 2b are images acquired along the basal artery in the absence and presence of pumping power. Because this region is relatively close to the surface, the basal artery and its junction point with vertebrate arteries can be directly observed by the external coil (Fig. 2a). But the smaller vessel that horizontally passes through the center region of the basal artery is not well-defined. When the pumping power is turned on, vascular boundaries of the basal artery become much clearer, and the continuous lumen of the horizontal vessel is easily identifiable (Fig. 2b).
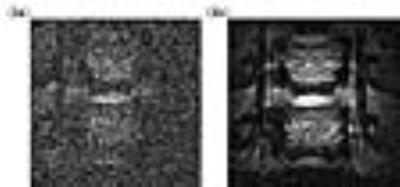
Figs. 3a and 3b are images acquired along the cervical vertebrate arteries with the pumping power turned off and on respectively. As shown in Fig. 3a, the anatomical contour is barely visible in the noisy background when this deep lying region is detected by the external surface coil. In contrast, the amplified image (Fig. 3b) has much better views of vertebrate arteries whose boundaries can be clearly identified along the cervical spine.
Conclusion
An endo-esophageal Wireless Amplified NMR Detector has been constructed to improve the imaging quality of the basal artery and vertebrate arteries. In addition to vessel wall imaging, this detector could be used to characterize inflammation or carcinogenesis in thyroid and lymph nodes. It may also eliminate the need for hard-wired connections normally used in endo-vascular2, endo-rectal3 or intraoral coils4,5, thus improve their operation flexibility.Acknowledgements
The authors would like to thank Dr. Alan Koretsky and Dr. Joe Murphy-Boesch for their inspirations from the initial stage of this project. This research is currently supported by the NIBIB under award number R00EB016753 and the Department of Radiology at Michigan State University. The content of this paper does not necessarily represent official views of the NIH.References
1. Qian CQ, Yu X, Chen DY, et al. Wireless Amplified Nuclear MR Detector (WAND) for High-Spatial-Resolution MR Imaging of Internal Organs: Preclinical Demonstration in a Rodent Model. Radiology 2013;268(1):228-236.
2. Atalar E, Bottomley PA, Ocali O, et al. High resolution intravascular MRI and MRS by using a catheter receiver coil. Magn Reson Med 1996;36(4):596-605.
3. Schnall MD, Lenkinski RE, Pollack HM, et al. Prostate - Mr Imaging with an Endorectal Surface Coil. Radiology 1989;172(2):570-574.
4. Li R, Bishop P, Jesmanowicz A, et al. Improving whole brain coverage and Signal-to-Noise ratio using novel intra-oral and over head surface coil array in rat under 9.4T. Proc Intl Soc Mag Reson Med 2011:1822.
5. Idiyatullin D, Corum CA, Nixdorf DR, Garwood M. Intraoral Approach for Imaging Teeth Using the Transverse B-1 Field Components of an Occlusally Oriented Loop Coil. Magn Reson Med 2014;72(1):160-165.
Figures