2677
A new human-scale fast field-cycling MRI system for clinical applications1Aberdeen Biomedical Imaging Centre, University of Aberdeen, Aberdeen, United Kingdom
Synopsis
Fast-field cycling MRI is a novel technique that involves cycling the main magnetic field during image acquisition. By doing this, information on the magnetic field dependence of parameters such as the T1 relaxation time can be investigated and exploited as a new form of endogenous image contrast. In this abstract we present progress on a new human-scale fast field-cycling MRI system with a detection field of 0.2 T.
Purpose
Fast Field-Cycling MRI1 (FFC-MRI) is a novel MRI technique in which the external magnetic field is switched during the imaging experiment. By doing this, FFC-MRI grants access to information which is invisible to conventional MRI scanners, including the variation of T1 with magnetic field. These measurements, known as T1-dispersion, exhibit great promise as a new form of endogenous image contrast, and may have application in the early diagnosis of a range of diseases including osteoarthritis2, cancer and neurodegeneration. Furthermore, T1-dispersion at ultra-low magnetic fields (less than 10 kHz proton Larmor frequency), measured by FFC-MRI, can offer new insight into the molecular dynamics and structure of tissues and is a largely unexplored area of study in in-vivo imaging. The construction of an MR imaging system capable of rapidly switching magnetic fields, and reaching ultra-low fields requires novel magnets, power supplies and control electronics. Here we describe progress on a new whole-body human sized FFC imaging system and present images obtained from normal volunteers.Methods
The magnet (Tesla Engineering Ltd, Storrington, UK) is of a resistive design with a length of 2 m and an inner bore diameter of 500 mm. The main magnet is comprised of three identical coils, angularly offset from each other by 120° embedded in epoxy resin. The magnet includes three conventional gradient coils and eight shim coils, which provide shimming up to 4th order. The current supplied to the shim coils is magnetic field dependent to ensure optimum shimming at all field values. Each of the B0 coils is driven by a rack of 6 current amplifiers (IECO, Helsinki, Finland). Each amplifier rack is capable of supplying a maximum current of 600 A, so the total current supplied to the magnet is 1800 A, corresponding to a maximum field strength of 0.2 T (8.52 MHz proton Larmor frequency). The magnet power supply incorporates a zero-flux current tranducer (Danfysik A/S, Denmark) in a feedback loop which is configured for a current stability of approximately 1 ppm. The system can switch between zero and maximum field in 20 ms, corresponding to a maximum dB/dT of 10 T/s. The scanner is also equipped with a set of three orthogonal 2-metre wide square Helmholtz coils (Figure 1) centred on the isocentre of the magnet to provide earth’s field cancellation, allowing a minimum B0 of less than 1 μT (42 Hz) to be achieved over a 30 cm DSV. The gradients and RF system are controlled by a commercial MRI console (MR Solutions Ltd, Guildford, UK) while the main magnet coil, shim coils and earth-field cancellation coils are controlled by a dedicated computer running in-house software written in Labview (National Instruments, Austin, US). The main magnetic field is set and controlled by a 16-bit, high-precision DAC which provides a field resolution of 3 μT. Synchronisation between the main console and the Labview-controlled PC is accomplished using TTL lines with timing accuracy on the order of 100 ns.Results
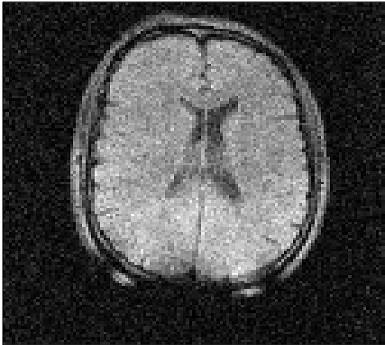
The system has been fully commissioned and is now capable of in-vivo imaging using its full field range. Figure 2 shows an example of an image of the head of a human volunteer obtained at an evolution field of 100 mT.Discussion
The novel system design described here will allow us to explore the unique T1 dispersion contrast made available by FFC-MRI in greater detail than was possible using our previous system3 which had detection at 0.06 T. Furthermore, the use of a purely resistive magnet design allows us to access ultra-low magnetic fields along with the associated information on slow molecular dynamics. Future work will concentrate on identifying how this newly accessible region of the T1 dispersion curve can be exploited for clinical diagnosis.Acknowledgements
We acknowledge support for this project from the European Union, under Horizon-2020 project 668119, “IDentIFY”References
1. Lurie, D. J. et al. Fast field-cycling magnetic resonance imaging. Comptes Rendus Phys. 11, 136–148 (2010).
2. Broche, L. M., Ashcroft, G. P. & Lurie, D. J. Detection of osteoarthritis in knee and hip joints by fast field-cycling NMR. Magn. Reson. Med. 68, 358–362 (2012).
3. Lurie, D. J., Foster, M. A., Yeung, D. & Hutchison, J. M. S. Design, construction and use of a large-sample field-cycled PEDRI imager. Phys. Med. Biol. 43, 1877–86 (1998).