2669
Multiple-Mouse Magnetic Resonance Imaging with CryoProbes1Mouse Imaging Centre, Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Ontario Institute for Cancer Research, Toronto, ON, Canada
Synopsis
Multiple-mouse MRI was implemented on a 7-Tesla magnet with four Cryogen (liquid helium)-cooled radio frequency probes. The high signal-to-noise ratio offered by the probes enabled spatial resolutions of 60 μm isotropic in <2 hours, a significant improvement for in vivo anatomical imaging of the mouse brain. Several 3D pulse sequences including FLASH/MP2RAGE/RARE were implemented for multiple-mouse acquisition. Manganese-enhanced T1-weighted images were obtained at two different resolutions of 60 μm and 75 μm. Results demonstrate the combined benefits of cryogen-cooled coils and multiple-mouse MRI.
Introduction
Owing to the high genetic similarity with humans, along with practical considerations of cost, rapid developmental, and availability of genetically-engineered lines, the mouse is a preferred model of human disease. Mouse MRI is a powerful tool for investigation of these models, enabling quantitative comparison between groups and direct phenotype comparisons with humans.
Structural MRI of the mouse demands resolutions of ~100 μm or better for comparable “anatomic resolution” to human MRI (representing a ~10-fold increase in each linear dimension). The increased demands for resolution comes at the cost of signal-to-noise ratio (SNR). This cost is generally offset by higher magnetic field, purpose-built room-temperature radiofrequency (RF) coils, and longer scan times. The latter, however, comes with the compromise that mouse MRI is slower than the human equivalent. This problem is further compounded by the fact that mouse studies frequently require 10 or more animals in multiple experimental groups, possibly at multiple time points. Even simple study designs may require hundreds or thousands of mouse MRI scans1. To address this throughput issue, multiple-mouse MRI was developed2, allowing imaging of up to 16 mice simultaneously. More recently, the SNR cost of mouse MRI has also been addressed by design of cryogen-cooled RF probes (CryoProbes), in which both the RF coil and preamplifier are cooled with liquid helium to reduce the operating temperature and eliminate electronic noise3,4. As electronic noise is comparable in magnitude to sample noise in a typical room-temperature RF coil, the CryoProbe provides a substantial gain in SNR for mouse MRI, which may be used for increased resolution or decreased scan time.
In the current work, we combine the benefits of multiple-mouse MRI and CryoProbes on a 7-Tesla magnet.
Methods
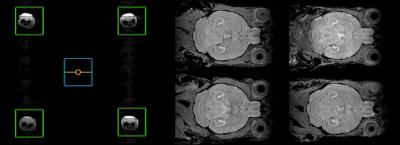
The multiple-mouse MRI setup with CryoProbes was implemented on a 30 cm Bruker magnet equipped with an Avance III HD console. The gradient system (BFG 20S) had 20 cm inner diameter and strength 300 mT/m. Four CryoProbes were mounted two-by-two on a ~8.2 cm center-to-center square. The coils were driven with a single RF transmitter split four-ways (and then in quadrature to a total of eight coil elements). Eight separate receivers were used to digitize the resulting signal for reconstruction.
We assayed several 3D in vivo imaging sequences, including a manganese (Mn)-enhanced FLASH, Mn-enhanced MP2RAGE, and a 3D RARE sequence. In each case, a “cylindrical” k-space acquisition was used, in which only phase-encode points on the Cartesian grid that lie within a prescribed radius were collected (eliminating the corners of k-space and providing ~25% decrease in scan time), a practice in standard use at our site.
Results
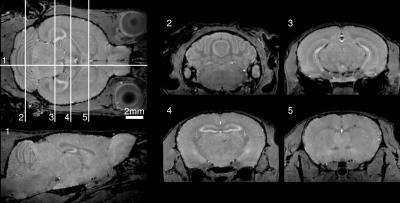
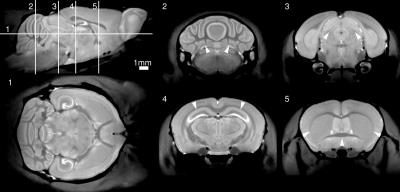
The bulk of imaging to-date on the multiple-mouse MRI CryoProbe system has been acquired with the Mn-enhanced FLASH sequence at isotropic resolutions of either 60 μm (parameters: TE/TR=8/32 ms, excitation 26°, FOV 25x22x22 mm, NA=2, 112 min acquisition time) or 75 μm (parameters: TE/TR=8/26 ms, excitation 23°, FOV 25x22x22 mm, NA=2, 59 min acquisition time). Sample slices from an individual 3D volume and from a set of four mice acquired simultaneously are provided in Figures 1 and 2. An average image composed of 60 mice is shown in Figure 3. Alternative image contrasts were tested, including an MP2RAGE acquisition and a 3D RARE acquisition.Discussion
Our results indicate the feasibility of multiple-mouse MRI with CryoProbes. In comparable in vivo imaging using an older room-temperature coil array installed on our 7-Tesla Agilent system, we routinely acquire Mn-enhanced images at 90 μm in 90 mins, 7-at-a-time. The demonstrated operation of the multiple-mouse MRI CryoProbe system shows enhanced resolution with slightly lower throughput (8 mice at 75 μm in 120 mins, or 4 mice at 60 μm in 90 mins). Alternatively, increased throughput (8 images in 82 mins) at higher SNR could be achieved at an equivalent resolution of 90 μm. The combined benefits of multiple-mouse MRI and CryoProbes will provide new opportunities for high-throughput characterization of mouse models of human disease.Acknowledgements
We would like to thank Ramy Ayoub and Shannon Egan for their help in mouse handling. We are grateful for the financial support from the Canada Foundation for Innovation, the Ontario Research Fund, and the Ontario Institute for Cancer Research.References
1. Ellegood J, Henkelman RM, and Lerch JP. Magnetic Resonance Imaging as a Tool for the Study of Mouse Models of Autism. Autism 2012.
2. Bock NA, Nieman BJ, Bishop JB, and Henkelman RM. In Vivo Multiple-Mouse MRI at 7 Tesla. Magnetic Resonance in Medicine. 2005, 54:1311–1316.
3. Baltes C, Radzwill N, Bosshard S, Marek D, Rudin M. Micro MRI of the mouse brain using a novel 400 MHz cryogenic quadrature RF probe. NMR in Biomedicine 2009, 22(8):834-842.
4. Ratering D, Baltes C, Nordmeyer-Massner J, Marek D, Rudin M. Performance of a 200-MHz cryogenic RF probe designed for MRI and MRS of the murine brain. Magnetic Resonance in Medicine 2008, 59(6):1440-1447.
Figures