2660
An adjustable 8-channel receive coil for population studies of marmosets1Centre for Functional and Metabolic Mapping, The University of Western Ontario, London, ON, Canada
Synopsis
An eight-channel RF coil was developed for imaging the common marmoset at 9.4T. The coil was adjustable in width to accommodate different head sizes while maintaining high SNR, thereby facilitating the study of larger cohorts of animals. Tuning and matching of the coil did not require adjustment over the range of potential head sizes. EPI time series were acquired, showing minimal geometric distortion with a two-fold reduction factor. A two-fold reduction factor could be achieved in both the left-right and anterior-posterior directions.
Purpose
A New World monkey, the common marmoset (Callithrix jacchus) is becoming a useful animal model for pharmaceutical and transgenic studies1,2. Despite this, dedicated RF coils for marmoset imaging are relatively rare; most studies have relied on volume coils3 or single surface coils4. More recently, dedicated multi-channel arrays have been custom-made for individual animals5,6. This design produces high SNR and is compatible with functional imaging, but is potentially impractical for studies of larger populations. This abstract describes an 8-channel receive array that is adjustable in width to accommodate a range of head sizes.Methods
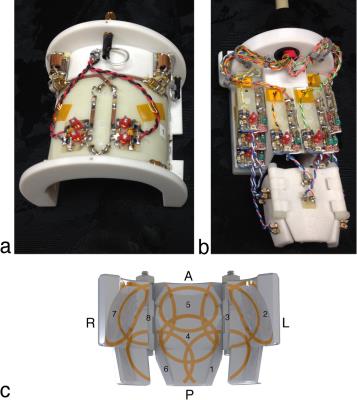
Three-dimensional MRI scans were used to create a CAD model of a marmoset head. The head model was smoothed to make it more generic and split into three pieces (with two hinges) to allow it to be adjustable in width. The former could accommodate head widths ranging from 37 mm to 50 mm. Eight elliptical elements, milled from 1/2-ounce copper adhered to 130-μm thick fiberglass, were adhered to the inner surface of the former. Inferior elements were larger in diameter—this produced a larger penetration depth to compensate for the increased distance to the brain caused by the lateral muscles. Coil elements that spanned the hinged gaps were bridged with “pig-tailed” wires to allow them to expand and contract as the former changed shape. Active-detuning circuitry, preamplifier decoupling circuitry, a fast-blow fuse, and a tuning capacitor were placed directly anterior to the coil. Low-input-impedance preamplifiers were placed outside the scanner bore (approximately 113 cm away) due to space constraints within the 12-cm-diameter bore. All coils were tuned to 400 MHz. A photograph of the coil is shown in Fig. 1.
All imaging was performed on a 9.4T horizontal-bore animal MRI scanner with a 12-cm-diameter gradient coil. A two-channel overlapped coil (each element measured 8.2-cm wide by 6.9-cm long) was used for RF transmission, with RF shimming applied over the whole head prior to image acquisition. Temporal SNR maps were generated from an EPI time series (resolution: 500 μm isotropic, TE/TR: 15/1500 ms, number of slices: 40, bandwidth: 312 kHz, flip angle: 35°). Geometry factor maps were created from fully sampled gradient-echo images (FOV: 48 x 48 mm, bandwidth: 50 kHz) of a marmoset head-shaped phantom. K-space data was under-sampled retrospectively and reconstructed using SENSE7. The noise correlation was derived from a bandwidth-matched, noise-only free-induction decay.
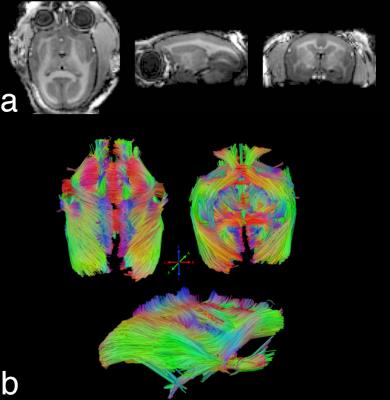
To demonstrate image quality, an MP2RAGE image was acquired (TE/TR: 1.9/6.1 ms, TI1/TI2: 900/2900 ms, BW: 25 kHz, flip angle: 4°/5°, number of averages: 4, acquisition time: 20 min 48 s). A fiber tracking image was acquired with a single-shot diffusion-spectrum imaging scheme (resolution: 600-μm isotropic, number of encoding directions: 256, b-value: 1000 s/mm2, number of averages: 2). Preprocessing included motion and eddy current correction, followed by diffusion model fitting and deterministic fiber tracking8.
Results and Discussion
The tune and match of the transmit coil did not change with changing width of the receive coil. After RF shimming, the transmit coil achieved a flip-angle uniformity of 16% over the brain (i.e., excluding the skull, skin, and lateral muscles).
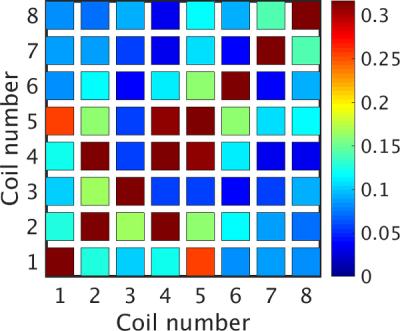
Geometric overlap of receive elements and preamplifier decoupling were capable of producing a mean and maximum noise correlation of 12% and 32%, respectively (Fig. 2). The Q-ratio of an isolated receive element was 1.8. The mean S11 of the eight receive elements was -30 dB. When the former was varied from its minimum to maximum width, the four bridged elements had a mean frequency shift of 1.5 MHz. The resulting worst-case S11 was still sufficient (-15 dB at 400.2 MHz). The impedance at the shifted resonant frequency remained at 50 Ω.
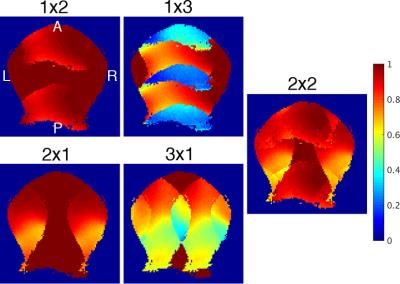
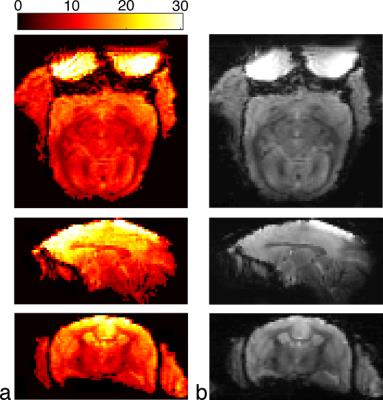
Inverse geometry maps (Fig. 3) demonstrate that a two-fold reduction rate can be achieved in both the left-right and anterior-posterior directions with less than a 20% increase in reconstruction noise. This parallel acceleration allows for reduced geometric distortion, as seen in the EPI images of Fig. 4. The corresponding temporal SNR maps show high SNR at the superior aspect of the brain with a moderate decrease in the temporal and occipital lobes—this is caused by the larger distance between the coil and brain in these regions, as dictated by the lateral muscles, neck, and shoulders. The resultant image quality is demonstrated in Fig. 5.
Conclusions
A receive coil was developed that was capable of adjusting to the size of the marmoset head. This allowed for a consistent loading factor to be maintained (and therefore SNR), with varying marmoset head size, using the same coil. The design, therefore, facilitates the study of larger cohorts of animals.Acknowledgements
The authors would like to thank Nicole Hague and Ashley Kirley for animal care and preparation and Dr. Alex Li for scanning assistance.References
1. Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Seminars in Fetal & Neonatal Medicine 2012;17(6):336-340.
2. Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature 2009;459(7246):523-U550.
3. Yamazaki Y, Hikishima K, Saiki M, Inada M, Sasaki E, Lemon RN, Price CJ, Okano H, Iriki A. Neural changes in the primate brain correlated with the evolution of complex motor skills. Scientific Reports 2016;6.
4. Sadagopan S, Temiz-Karayol NZ, Voss HU. High-field functional magnetic resonance imaging of vocalization processing in marmosets. Scientific Reports 2015;5.
5. Papoti D, Yen CCC, Mackel JB, Merkle H, Silva AC. An embedded four-channel receive-only RF coil array for fMRI experiments of the somatosensory pathway in conscious awake marmosets. NMR Biomed 2013;26(11):1395-1402.
6. Papoti D, Yen CC, Hung CC, Ciuchta J, Leopold DA, Silva AC. Design and implementation of embedded 8-channel receive-only arrays for whole-brain MRI and fMRI of conscious awake marmosets. Magn Reson Med 2016.
7. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 1999;42(5):952-962.
8. Yeh
FC, Verstynen TD, Wang YB, Fernandez-Miranda JC, Tseng WYI. Deterministic
diffusion fiber tracking improved by quantitative anisotropy. Plos One
2013;8(11).
Figures