2624
Evaluation of a thermal dose based safety concept for 7T pTx coils1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany
Synopsis
We propose a straightforward procedure to calculate thermal dose values (CEM43) from upper limit values of tissue temperatures utilizing T-matrix formalism. The procedure is evaluated for a 7T 8-channel transmit/receive (pTx) head coil using the RF power data of a long duration neuroscience MR session and a scenario when heating of eye tissue is worst. The proposed approach, as a generalization of the thermal dose concept for body coils, is capable to determine reliably the risk of excessive tissue heating when using pTx coils.
Purpose
A simple procedure is proposed to calculate thermal dose values (CEM43) from upper limit values of tissue temperatures utilizing a T-matrix formalism. In case of equal maximum forward power for each RF channel this calculation scheme is independent of actual RF phase settings providing a robust safety measure. The procedure is evaluated for an extended neuroscience MR session and a pTx scenario when heating of eye tissue is worst, i.e. the steady-state eye temperature is just below the corresponding upper limit value. The proposed approach, as a generalization of the thermal dose concept, is capable to determine reliably the risk of excessive tissue heating when using pTx coils.Methods
Coil model: Shielded decoupled loop array1,2 with nch=8 loops operated at 300 MHz using ‘Duke’ as head model. Tissue parameters were taken from the IT'IS database3.
Thermal modelling: Pennes' Bioheat Equation4 (PBE) was implemented utilizing a numerically stable GPU code5.
Determination of tissue specific upper limit temperatures: The calculation of the T-matrix Tij(x,y,z) requires nch2 = 64 PBE simulation runs. Steady-state temperatures are given for each arbitrary voltage vector |u> with total forward power P0 = <u|u>/8Z0 by T(x,y,z)= <u|Tij(x,y,z)|u> + T0(x,y,z) with T0(x,y,z) being steady-state temperatures without external power deposition. Tissue temperatures T(x,y,z) are always less than Tul(x,y,z) = Σij|Tij(x,y,z)|/nch + T0(x,y,z) if the forward power for each RF channel is limited to P0/nch 6.
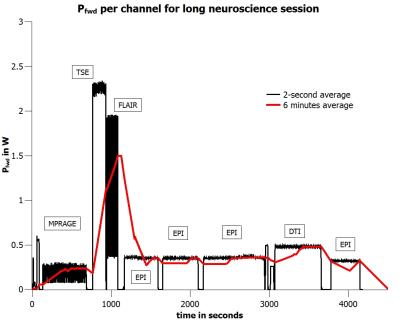
Time course of forward power: For evaluation purposes we used the measured time course (2s average) of a 70-min neuroscience MR session (Fig.2). The time course was scaled to obtain 6-minute averages of forward power of either <1.5W or <3W per channel. With a forward power of <1.5W per channel the coil is just in compliance1,2 with the 20W/kg limit of psSAR10g 7.
Voltage vector for worst case eye heating: The eigenvector of VOP#34 out of 500 VOP's calculated from 3D Q-matrices5 was found to produce maximal eye temperatures5. When setting the forward power per channel to 1.5 W and keeping the phase settings unchanged the steady state temperature of eye tissue is 40.37°C compared to the corresponding upper limit value of 40.46°C (Fig.1).
Determination of tissue specific thermal doses: During the PBE simulations global temperature maxima were determined (every 2s) for the tissue groups muscle-skin-fat (pTMSF), central nervous system (pTCNS), bone and cartilage (pTBC) and eye tissues (pTEYE). In addition, and similar to Ref. 8, a simplified, computational much less demanding model was developed by applying a linear convolution kernel to calculate peak tissue temperatures using a decay time of 600s and an amplitude corresponding to the tissue specific upper limit values. CEM43 values (thermal doses) were then calculated according to Ref. 4.
Results and Discussion
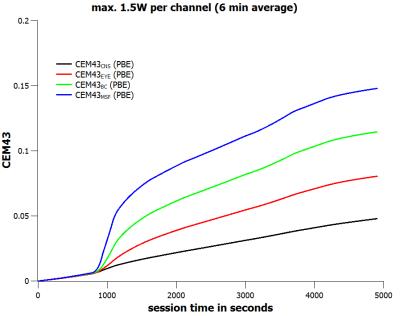
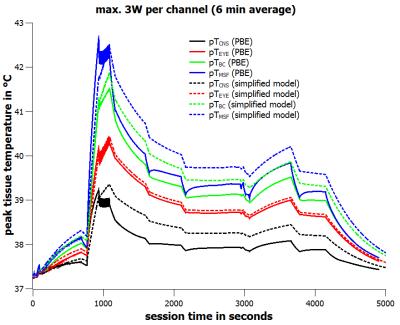
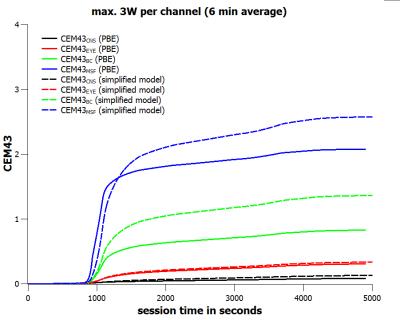
For a forward power of 1.5W per channel, i.e. a total forward power of 12 W, the tissue specific peak steady-state temperatures are limited to pTCNS ≤ 39.37°C, pTEYE ≤ 40.46°C, pTBC ≤ 41.88°C, and pTMSF ≤ 42.49°C (Fig.1). For a special voltage vector designed for worst-case eye heating the steady-state eye temperature is just below the corresponding upper limit value (Fig.1). When applying the time course of the extended neuroscience MR session (Fig.2) with a maximum forward power of 1.5W per channel (6-minute average) the final thermal doses are CEM43CNS ≤ 0.048 min CEM43EYE ≤ 0.080 min, CEM43BC ≤ 0.114 min, and CEM43MSF ≤ 0.148 min, respectively (Fig.3). These thermal doses are much lower than threshold values4 of 2 minutes (CNS, EYE), 15 minutes (BC), and 41 minutes (MSF). When doubling the input power, i.e. the 20W/kg limit of psSAR10g is exceeded by a factor of two, the final thermal doses are CEM43CNS ≤ 0.084 min, CEM43EYE ≤ 0.307 min, CEM43BC ≤ 0.828 min, and CEM43MSF ≤ 2.078 min, respectively (Fig.5). Again, CEM43 values are well within safe limits. Since the simplified model reasonably overestimates tissue temperatures (Fig.4) the corresponding thermal dose values are overestimated as well (Fig.5).Conclusion
We introduced a simple procedure to calculate thermal dose values (CEM43) for multichannel transmit coils utilizing a T-matrix formalism for calculation of upper limit values of tissue temperatures. Together with a single heuristic model parameter the procedure reasonably overestimates tissue temperatures and CEM43 values. In this way a reliable thermal dose monitor can be established for pTx coils. Since no surrogate parameters like psSAR10g were used as safety measures, collateral risks - associated e.g. with external dielectric pads or metallic implants - can be assessed consistently within the proposed concept.Acknowledgements
No acknowledgement found.References
1. Seifert F, et al., Reliable and robust RF safety assessment of transmit array coils at ultrahigh fields, Proc. ISMRM 22 (2014) 4891.
2. Seifert F, et al., 7T 8-channel pTx head coil with high B1+ efficiency optimized for MRS, ISMRM 24 (2016) 3545.
3. Hasgall PA, et al., IT’IS Database for thermal and electromagnetic parameters of biological tissues. Version 3.0 (2015), doi: 10.13099/VIP21000-03-0. www.itis.ethz.ch/database.
4. Murbach M, et al., Thermal Tissue Damage Model Analyzed for Different Whole-Body SAR and Scan Durations for Standard MR Body Coils, MRM 71 (2014) 421-431.
5. Seifert F. et al., Correlation of psSAR and tissue specific temperature for 7T pTx head coils - a large scale simulation study, Proc. ISMRM 23 (2015) 380.
6. Seifert F. et al., From local SAR to thermal dose - towards robust safety concepts for UHF pTx coils, ISMRM Workshop on Ultra High Field MRI, flash poster #32 (2016) Heidelberg, Germany.
7. IEC60601-2-33:2010.
8. Neufeld E. et al., Rapid method for thermal dose-based safety supervision during MR scans, Bioelectromagnetics 36 (2015) 398-407.
Figures