2616
Simultaneous slice excitation for accelerated passive marker tracking via phase-only cross correlation (POCC) in MR-guided needle interventions1Dept. of Radiology, Medical Physics, Medical Center - University of Freiburg, Freiburg, Germany
Synopsis
Minimally invasive interventions benefit from image guidance during instrument positioning. To ensure high image quality, MR-guided interventions are preferably performed in closed bore systems using MR markers and passive tracking sequences to localize and monitor the interventional device. In this work we accelerate a passive marker tracking sequence designed for needle-guided procedures using a simultaneous multi-slice excitation. With this technique the acquisition time is reduced by 33%, which results in a higher temporal fidelity while maintaining targeting accuracy.
Introduction
MRI offers an excellent soft tissue contrast and enables functional imaging which makes MRI highly favorable for guidance of interventional procedures. Interventions, e.g. needle biopsies, require a high image quality and are therefore preferably performed in closed-bore MR-systems. Unfortunately, such systems offer no direct line of sight and severely limit patient access. To overcome this limitation and to facilitate MR-guided interventions, passive MR markers are used for instrument localization. To automatically follow these passive markers, a pulse sequence based on a phase-only cross correlation (POCC) algorithm1 was introduced which acquires cross-sectional images of the marker for localization in combination with an in-plane view for targeting. To accelerate the localization part of the POCC sequence we here introduce a simultaneous multi-slice excitation technique (POMP2). The accelerated sequence is evaluated in phantom measurements during needle insertions and compared to the existing tracking approach relying on serial acquisitions.Methods
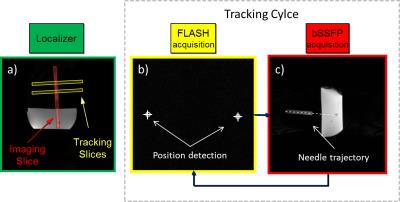
The POCC tracking sequence1-3 detects the position of a passive, cylindrical marker filled with contrast agent and features a central hole for needle insertions. In its current implementation, the sequence tracks the marker’s position and orientation from two parallel slices (T1-weighted FLASH) oriented perpendicular to the marker’s symmetry axis so that the marker appears in a ring-like cross section. The position of the ring is automatically detected via a POCC algorithm and the calculated position information is used to subsequently align a targeting image (bSSFP) parallel to the marker’s symmetry axis, i.e. the planned needle trajectory. The tracking and targeting images are continuously acquired to automatically follow manipulations of the marker in real-time.
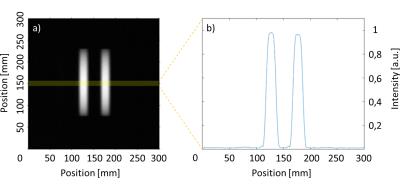
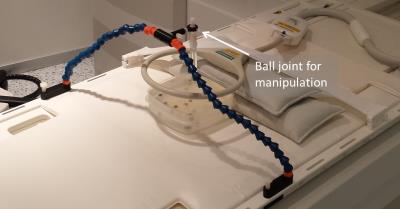
In this work the two parallel tracking slices are acquired simultaneously (i.e., acceleration of the tracking part by a factor of 2) by using the simultaneous acquisition method POMP2. The POMP excitation pulse is calculated from the superposition of two slice selective sinc-shaped pulses in the time domain, in which the phase of one of these pulses is modulated with a factor of $$$exp(-iφt) (φ=2πγGΔz; γ:$$$ gyromagnetic constant, $$$G:$$$ slice selection gradient). The phase modulation induces a spatial shift $$$Δz$$$ between the two slices. The signals from both slices are separated in the image domain by an additional ±180° phase-cycling which shifts the signal from one slice in phase encoding direction. POMP excitation (pulse duration = 0.8ms, time-bandwidth product = 1.6) was implemented on a 1.5T system (Siemens Symphony) and the slice profiles were measured in a homogeneous phantom (Fig. 1). Afterwards, the POMP pulse was inserted into the POCC tracking sequence (Fig. 2) replacing the conventional serial acquisition of the two tracking slices. The two versions of the tracking sequence were then compared during needle insertions in a 2% agarose gel that contained 24 fiducial targets (diameter: 7.8±0.6mm). A customized marker holder fixed to the patient table was used to manually manipulate the marker via a ball joint (Fig. 3). To compare both sequences the following experimental work-flow was done for each target:
(i) positioning of the marker in a center (i.e. neutral) position,
(ii) online targeting with either the existing (TA=2.3s for one cycle) or the POMP-based (TA=1.5s for one cycle) tracking sequence until the planned needle trajectory was aligned with the target (the two sequences were alternated with every other target),
(iii) needle insertion into the target under HASTE guidance using the planned needle pathway according to the respectively preceding tracking sequence.
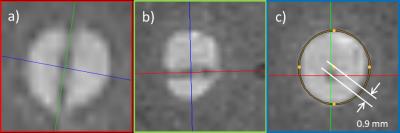
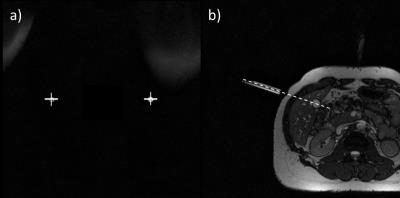
The targeting duration and the lateral distance of the needle pathway to the target’s center point using reformatted views of high-resolution data set (3D GRE, 0.6×0.6×0.6mm3) were measured for each target (Fig. 4a-c). Finally, the sequence was tested in vivo by targeting different structures in the abdomen of a volunteer.
Results
The integration of the POMP scheme reduces the duration of the total image acquisition cycle (tracking and imaging) by 33% while maintaining the desired slice selectivity (Fig. 1). In the phantom experiment, all targets were successfully punctured with a mean targeting time of 82±35s (mean ± standard deviation) and a lateral targeting accuracy of 1.6±0.7mm for the POMP version of the POCC sequence, and 100±36s and 1.5±0.8mm for the serial POCC sequence, respectively. Proof-of-concept of the in vivo applicability of the sequence could be successfully demonstrated (Fig. 5).Discussion & Conclusion
Our experiments demonstrate that
simultaneous slice acquisition via POMP was successfully integrated into the
POCC tracking sequence for percutaneous needle interventions. The phantom results
indicate that accelerated marker tracking translates into a shorter targeting
procedure without degrading the puncture accuracy. The accelerated sequences
might be a valuable tool to further reduce durations of POCC-guided
interventions.
Acknowledgements
We would like to thank Dr. Florian Maier (German Cancer Research Center (DKFZ), Heidelberg, Germany) for support with the implementation of the tracking sequence, and grant support from the DFG under grant number HA 7006/1-1.References
1. de Oliveira A, et al. Automatic passive tracking of an endorectal prostate biopsy device using phase-only cross-correlation. Magn Reson Med 59:1043–1050 (2008)
2. Krafft AJ, et al. Passive marker tracking via phase-only cross correlation (POCC) for MR-guided needle interventions: Initial in vivo experience. Physica Medica 29:607-614 (2013)
3. Zamecnik P, et al. Automated Real-time Needle-Guide Tracking for Fast 3-T MR-guided Transrectal Prostate Biopsy: A Feasibility Study. Radiology 273:879-886 (2014)
4. Glover G H J. Phase-offset multiplanar (POMP) volume imaging: A new technique. Magn Reson Imag 1:457–461 (1991)
Figures