2615
2D UTE-based MR thermometry of frozen tissue: feasibility during in vivo MRI-guided cryoablation1Radiology and Nuclear Medicine, Radboud University Medical Centre, Nijmegen, Netherlands, 2Radiology - Medical Physics, Medical Center University of Freiburg, Freiburg, Germany, 3Urology, Radboud University Medical Centre, Nijmegen, Netherlands, 4MIRA Institute for Biomedical Engineering and Technical Medicine, University of Twente, Enschede, Netherlands
Synopsis
This study assessed the feasibility of 2D UTE-based MR thermometry of frozen tissue during in vivo MRI-guided cryoablation. Axial 2D UTE images were acquired at the end of the first and second freeze cycle during an MRI-guided renal cryoablation procedure. Measurable MR signal could be obtained from frozen tissue. MR temperature maps were estimated using a relative signal level calibration performed in ex-vivo porcine muscle. Our work demonstrates the feasibility of 2D UTE-based MR thermometry of frozen tissue during in vivo MRI-guided cryoablation, which could be an important step towards clinical application of this technique.
Introduction
MRI-guided cryoablation is a promising minimally invasive treatment with applications in liver, kidney, and prostate cancer1. To assure effective treatment, intraprocedural temperature feedback is desirable but a non-invasive approach is currently lacking. Previous studies have demonstrated measurable MR signal from frozen tissue using ultrashort TE (UTE) MR imaging2,3. Recently, we have shown the feasibility of 3D MR thermometry of cryoablation using UTE signal intensity in an ex-vivo setup4. In vivo, a 3D approach presents issues with out-of-volume magnetization due to non-selective excitation as well as undersampling to avoid unacceptably long measurement times. Therefore, this work investigated the feasibility of 2D UTE-based MR thermometry of frozen tissue during in vivo MRI-guided cryoablation.Methods
Calibration experiments
An MR-compatible cryoneedle (IceRod, Galil Medical) was inserted into ex-vivo porcine muscle specimens (n=2) at room temperature on a 3T clinical MR system (Magnetom Skyra, Siemens). Three fiber-optic probes (T1, Neoptix) were placed at 5mm intervals for temperature reference (Figure 1). Two freeze-thaw cycles of 10:3 min. were applied under continuous MR imaging using a single-slice dual echo UTE sequence that utilizes half pulse RF excitation followed by a center-out radial readout to achieve a shortest TE of 50µs. Imaging parameters included TR/TE1/TE2=32.4/0.05/1.93ms, flip angle=20°, slice thickness=10mm, FOV=360mm, matrix size=192x192, no. of spokes=384, averages=3 and acq. time=1:14min. Fat saturation was used and spatial saturation bands were placed symmetrically on both sides of the imaging slice to suppress out-of-slice magnetization5. Per temperature probe, signal intensity (SI) values of the first echo image were recorded for three voxels at same radial distance from the cryoneedle, normalized to the maximum signal level observed in cooled unfrozen tissue directly adjacent to the ice-ball and related to recorded temperatures. Data for temperatures <0°C were fitted by a mono-exponential function.
In vivo measurements
2D UTE imaging was performed during an MRI-guided renal cryoablation procedure on the same 3T MR system. After percutaneous placement of three cryoneedles, two 10:3 min. freeze-thaw cycles were applied under regular MR monitoring using T2-weighted half-fourier single-shot turbo spin echo (HASTE) imaging. Axial 2D UTE images with same measurement parameters regarding TR, TE and flip angle as in the calibration (matrix size reduced to 128x128) were acquired during the first minute of freezing and at the end of the first and second freeze cycle. For frozen tissue, signal values of the first echo image were normalized to the maximum signal level in cooled but unfrozen surrounding tissue. MR temperature maps were estimated based on the ex-vivo calibration curve.
Results
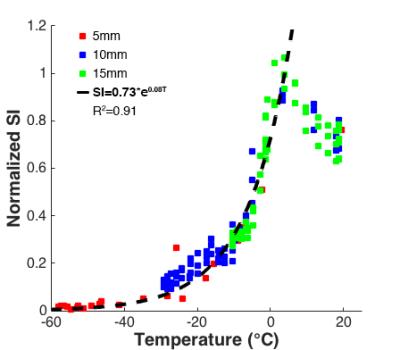
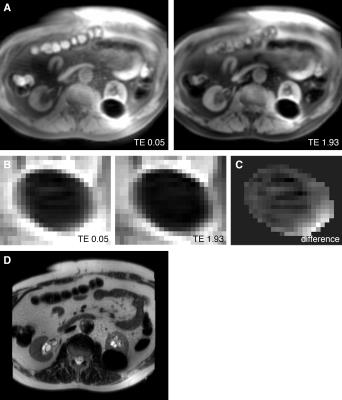
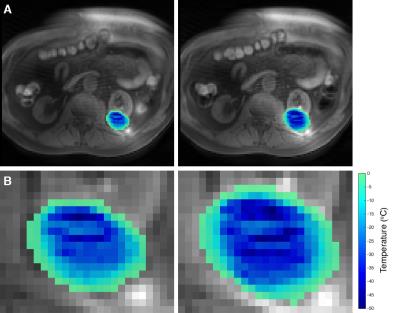
In the calibration experiments, normalized SI decreased mono-exponentially with temperature for T<0°C, with the signal decay fitted by SI=0.73*e0.08T (R2=0.91) (Figure 2). In vivo, measurable signal was observed within the ice-ball in the shortest TE images (Figure 3a-b). Signal within the frozen tissue is highlighted in the difference image between the first and second echo (Figure 3c). Estimated temperature maps calibrated to the ex-vivo measurements show temperature differences within the frozen zone at the end of the first and second freeze cycle (Figure 4).Discussion
This work demonstrates the feasibility of obtaining measurable MR signal from frozen tissue during in vivo cryoablation using 2D UTE imaging. Image acquisitions were achieved during free-breathing, within a clinically realistic window (<1min.) and with acceptable spatial resolution (2.8x2.8mm). Although a higher resolution would be preferable to reduce partial volume effects of the temperatures, a larger voxel size was chosen to optimize signal-to-noise in the frozen tissue. Estimated temperature maps were calculated based on relative signal level calibration in porcine muscle. A previous study has found the temperature dependence of UTE MR signal to be consistent between heart muscle, kidney and liver tissue, which could potentially obviate the need for tissue-specific calibration3. Alternatively, R2* may be used for MR thermometry of frozen tissue2,3. However this would require acquisition of multiple images at different short TEs to accurately estimate the relaxation rate, which may be impractical in a clinical time frame. Multi-echo acquisitions are limited by minimum echo spacing and in our data the second echo did not contain sufficient signal to perform accurate T2* estimation. Finally, our images were affected by subtle streaking. Increased FOV may help to reduce these artifacts in future acquisitions. Imaging improvements and validation of our calibration to kidney and prostate tissue are currently under investigation.Conclusion
2D UTE-based MR thermometry of frozen tissue is feasible during in vivo MRI-guided cryoablation. Further work addressing the accuracy of the MR-based temperature estimates is required. Clinical application could allow insight into the effective treatment zone during MRI-guided cryoablation procedures.Acknowledgements
No acknowledgement found.References
1. Morrison et al. JMRI 2008
2. Wansapura et al. Acad Radiol 2005
3. Kaye et al. JMRI 2010
4. Overduin et al. JMRI 2016
5. Krafft et al. ISMRM 2015, abstract: 87
Figures