2614
Real-time lesion targeting during MRI-guided prostate biopsy using an iPad: feasibility and initial clinical evaluation1Radiology and Nuclear Medicine, Radboud University Medical Centre, Nijmegen, Netherlands, 2MIRA Institute for Biomedical Engineering and Technical Medicine, University of Twente, Enschede, Netherlands
Synopsis
Our study assessed the feasibility of a novel method for real-time lesion targeting during transrectal in-bore MRI-guided prostate biopsy using a tablet device inside the MR room. Real-time targeting was technically successful in all patients and allowed targeted biopsy of the cancer suspicious region without requiring additional needle guide adjustments in all but one patient. Prostate cancer (PCa) was found in 18 of 20 patients. Our initial clinical experience indicates a substantial decrease in biopsy and procedure time as compared to standard targeting, which could be valuable to increase clinical applicability of the technique.
Introduction
Multi-parametric magnetic resonance imaging (mpMRI) has evolved towards a mature imaging modality to detect PCa with high localization accuracy1,2. Consequently, MR image-guidance has been proposed in targeting biopsies towards cancer suspicious regions (CSRs). Several studies have demonstrated promising results using in-bore MRI-guided prostate biopsy, achieving high diagnostic performance with use of less biopsy cores3-5. Nevertheless, an important concern for the technique is that procedure times are considerably longer than those typically needed for transrectal ultrasound (TRUS)-guided biopsy6,7. A substantial part of the biopsy procedure consists of repeated adjustments of the transrectal needle guide in order to it with the target area, requiring the physician to walk in and out of the MR scanner room. To overcome this issue, we propose a novel method for real-time lesion targeting during MRI-guided prostate biopsy using planning software and a tablet device inside the MR-room. In this work, we assessed feasibility of this technique and provide an initial clinical evaluation.Methods
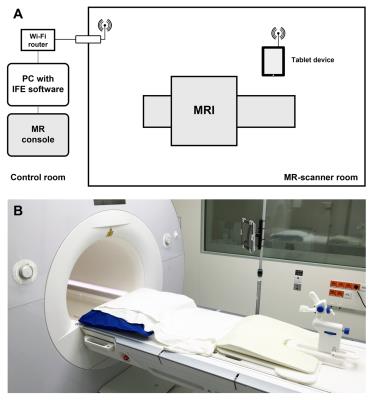
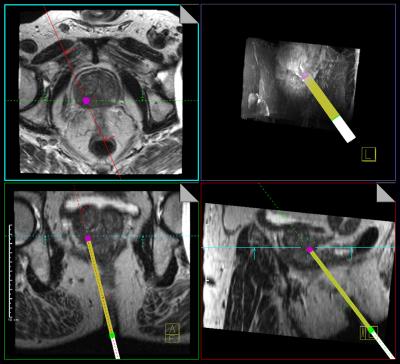
Prior to the study, MRI safety tests were performed according to the American Society for Testing of Materials (ASTM) standard test methods to identify the conditions to safely operate the tablet device inside the MR scanner room. Subsequently, twenty patients with one CSR with a PI-RADS score of ≥4 on diagnostic mpMRI who were scheduled for MRI-guided prostate biopsy were prospectively enrolled in this IRB-approved study. The technical setup is visualized in Figure 1. After reidentification of the CSR, two orthogonal imaging scan planes of an interactive real-time balanced steady-state free precession (BEAT) sequence (~3 imgs/s) were aligned to the needle guide pivoting point and target lesion using planning software (IFE, Siemens) (Figure 2). The physician then positioned the needle guide into both scan planes under real-time imaging feedback, with the MR images displayed on a tablet device (iPad 2, Apple) inside the scanner room. Technical feasibility of real-time lesion targeting was assessed as well as time to first biopsy, i.e. time between the last scan for lesion reidentification and confirmation scan of first biopsy, and total time of the biopsy procedure, i.e. time between the first and last MR scan of the biopsy procedure. A matched cohort that underwent MRI-guided prostate biopsy with the current standard method was retrieved from our institutional database for comparison. Mann-Whitney U-test was used to evaluate biopsy times between groups.Results
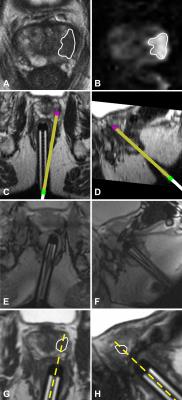
In all patients, real-time targeting was technically feasible without experiencing interferences or image artefacts due to the presence of the tablet device inside the MR-room. Targeting success was achieved in 19/20 patients (95%), requiring no additional needle guide adjustments to obtain the targeted biopsy after initial alignment under real-time imaging (Figure 3). In one patient, an additional adjustment step was needed because the lesion was located too anteriorly, outside of the maximum range of the needle guide. Using real-time targeting, mean time to first biopsy was 5.8±1.0 min and total procedure time was 23.7±4.1 min. In the reference cohort, this was 11.4±4.7 min and 33.9±7.5 min respectively. On average, time to first biopsy was 5.6 min (49%) faster (P<.001) and total procedure was reduced by 10.2 min (30%) (P<.001) using real-time targeting as compared to standard targeting. Mean manipulation time to align the needle guide to the lesion under real-time imaging was 1.1±0.3 min. Histopathological analysis of biopsy cores obtained using real-time targeting yielded prostate cancer in 18/20 patients (detection rate: 90%). Biopsy showed chronic prostatitis in the remaining two patients.Discussion
With this work we demonstrate the feasibility of a novel real-time method for lesion targeting during MRI-guided prostate biopsy. Alternative solutions to reduce biopsy times of transrectal MRI-guided prostate biopsy were previously proposed and have included robotic devices to aid in needle guide manipulation or the use of an automated needle-guide tracking sequence8,9. The proposed approach in this work is a relatively simple one. Although our feasibility study is limited to a small number of patients with a single CSR, we noted a marked reduction in procedure time compared to the standard biopsy procedure with the last ten procedures all performed in less than 25 minutes. Optimization of the planning software or integration with the scanner platform could further improve the workflow and facilitate implementation of the technique in clinical practice.Conclusion
Real-time lesion targeting was feasible during transrectal in-bore MRI-guided prostate biopsy. Our initial clinical experience indicates a substantial decrease in biopsy and procedure time as compared to current standard targeting.Acknowledgements
The authors would like to acknowledge Siemens AG Medical Solutions (Erlangen, Germany) for providing the IFE software as well as technical support.References
1. De Rooij et al. AJR 2014; 202(2):343–351.
2. Delongchamps et al. BJU Int. 2011; 107(9):1411–1418.
3. Hambrock et al. J Urol. 2010; 183(2):520–527.
4. Anastasiadis et al. Eur Urol. 2006; 50 (4):738–749.
5. Beyersdorff et al. Radiology. 2005; 234(2):576–581.
6. Irani et al. Br J Urol. 1997; 79(4):608–610.
7. Overduin et al. Curr Urol Rep. 2013; 14(3):209–213.
8. Bomers et al. Eur. Radiol. 2016; epub
9. Zamecnik et al. Radiology 2014; 273(3):879-86.
Figures