2613
MRI of Intra-Tumoral Iron-Oxide Labeled Clostridium novyi-NT Injections During Bacteriolytic Therapy in Solid Tumors1Northwestern University, Chicago, IL, United States
Synopsis
Bacteriolytic therapy with Clostridium novyi-NT has shown promise as a form of cancer treatment, but concerns remain regarding route of delivery and toxicity. Our study aimed to develop a method to visualize delivery with MRI during bacteriolytic therapy. We did so by incubating bacteria with iron oxide particles. In vitro viability studies and labeling efficiency studies were then performed, followed by phantom studies. Bacteria were then directly injected into rat liver tumors as well as mouse flank tumors and imaged. We demonstrate that the labeled bacteria can be directly visualized on MRI and is a reliable indicator of successful delivery.
Introduction
Bacteriolytic therapy as a form of cancer treatment has generated much attention in recent years 8. Clostridium novyi-NT was chosen specifically for its obligate anaerobic properties, which allows the bacteria to specifically colonize the necrotic cores of tumors. 1,5 Bacteriolytic therapy in conjunction with chemotherapy improved outcomes by destroying poorly perfused tumor cells that chemotherapy agents could not reach through the circulation, 3 and use of bacteria in conjunction with radiation therapy has enhanced the efficacy of previous treatment regiments by targeting severely hypoxic areas that were poorly treated with radiation. 2 Despite these findings, a major area of concern regarding bacteriolytic therapy is the possible toxicity that could accompany the presence of large numbers of bacterial spores injected into the bloodstream. 4 Therefore, a safer and more reliable method of bacterial delivery to the target site may be necessary before bacteriolytic therapy can be widely applied to the clinical environment. 6,7 The goal of our study was to use label Clostridium novyi-NT spores with iron-oxide for MRI visualization of intra-tumoral injection; these imaging methods should be valuable for intra-procedural optimization of tumor-directed administration procedures and early prediction of treatment outcomes.Materials and Methods
Iron Oxide Labeling: Vegetative state C. novyi NT were incubated with iron oxide particles coupled with rhodamine B (Ocean Nanotech, Inc.) at a concentration of 100µg/mL. Resulting bacteria underwent viability studies comparing germination rates as measured by optical density. Confocal microscopy was used to evaluate labeling efficacy of both vegetative bacteria and bacterial spores.
Phantom Studies: MRI phantoms were constructed by homogeneously mixing 5 x 108 spores with 250µL of 2% agarose. T2*-weighted images of the phantoms were acquired using a 7T MR scanner.
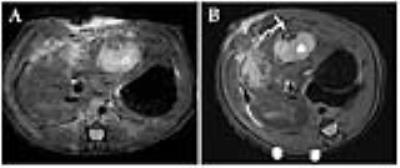
In vivo Studies: Direct injections of iron-oxide labeled bacteria in C57 mice with Panc02 flank tumors were performed for in vivo visualization with MRI. Animals were survived for 72 hours and T2 weighted images were obtained with a 7T MR scanner every 24 hours. Animals were terminated and gram staining was used to confirm presence of bacteria. In Sprague Dawley Rats, N1-S1 liver tumors were treated with direct injection of bacteria and imaged immediately after injection. Gram staining was used to confirm presence of bacteria.
Results
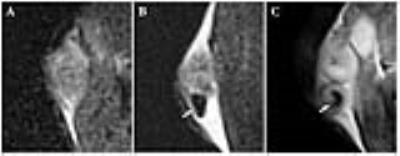
Labeled bacteria demonstrated a similar germination rate as control bacteria at 24, 48, and 72 hours of incubation and fluorescence microscopy demonstrated labeling efficacy of 100%. T2*-weighted imaging showed reduced signal in the labeled phantom (SNR 9.46±0.45) compared to unlabeled controls (SNR 22.3±1.93). In rat liver tumors, labeled bacteria could be visualized with MRI immediately after direct injection (Figure 1). Vegetative state bacteria were visible on histology in all successfully treated tumors. Interestingly, in the mouse model, tumors often proved too small for accurate direct intra-tumoral injection of spores (injection needle placement based upon simple digital palpation), thus leading to improper delivery of bacteria in some animals, and subsequent formation of an abscess adjacent to the tumor (Figure 2). In this case, labeling of the spores allowed for direct visualization of the injected bacteria, leading to an immediate indication of improper delivery.Discussion
C. novyi NT spores can be efficiently labeled with iron oxides by inducing sporulation in labeled vegetative state bacteria. Furthermore, the bacteria proved to be viable both in vitro and in vivo after labeling as supported by the viability studies along with the phantoms and follow-up studies in animal models. In vivo studies also suggest that iron-oxide labeling helps to provide an important confirmation of delivery and may also be a viable way to track bacterial redistribution and proliferation over the course of treatment.Conclusion
These methods have direct applications for image-guided bacteriolytic therapy and are permitting ongoing comparisons between IV and direct injection administration routes as well as the investigation of intra-arterial catheter-directed alternatives. In addition, visualization adds an extra layer of safety for potential future clinical applications.Acknowledgements
I would like to acknolwedge the members of Larson Lab and Northwestern University for the resources and research ideas. Imaging work was performed at the Northwestern University Cell Imaging Facility generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. MRI imaging was carried out at the Center for Translational Imaging.References
1. Agrawal N, Bettegowda C, Cheong I, Geschwind JF, Drake CG, Hipkiss EL, Tatsumi M, Dang LH, Diaz LA Jr, Pomper M, Abusedera M, Wahl RL, Kinzler KW, Zhou S, Huso DL, Vogelstein B. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci. 2004;101(42):15172-7.
2. Bettegowda C, Dang LH, Abrams R, Huso DL, Dillehay L, Cheong I, Agrawal N, Borzillary S, McCaffery JM, Watson EL, Lin KS, Bunz F, Baidoo K, Pomper MG,Kinzler KW, Vogelstein B, Zhou S. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc Natl Acad Sci. 2003;100(25):15083-8.
3. Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci. 2001;98(26):15155-60.
4. Diaz, L. A. Pharmacologic and Toxicologic Evaluation of C. Novyi-NT Spores. Toxicological Sciences 2005;88(2):562-75.
5. Maletzki C, Gock M, Klier U, Klar E, Linnebacher M. Bacteriolytic therapy of experimental pancreatic carcinoma. World J Gastroenterol. 2010;16(28):3546-52.
6. Roberts NJ, Zhang L, Janku F, Collins A, Bai RY, Staedtke V, Rusk AW, Tung D, Miller M, Roix J, Khanna KV, Murthy R, Benjamin RS, Helgason T,Szvalb AD, Bird JE, Roy-Chowdhuri S, Zhang HH, Qiao Y, Karim B, McDaniel J, Elpiner A, Sahora A, Lachowicz J, Phillips B, Turner A, Klein MK, Post G, Diaz LA Jr, Riggins GJ, Papadopoulos N, Kinzler KW, Vogelstein B, Bettegowda C, Huso DL, Varterasian M, Saha S, Zhou S. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci Transl Med. 2014;6(249):249ra111.
7. Staedtke V, Bai RY, Sun W, Huang J, Kibler KK, Tyler BM, Gallia GL, Kinzler K, Vogelstein B, Zhou S, Riggins GJ. Clostridium novyi-NT can cause regression of orthotopically implanted glioblastomas in rats. Oncotarget. 2015;6(8):5536-46.
8. Wong S, Slavcev RA. Treating cancer with infection: a review on bacterial cancer therapy. Lett Appl Microbiol. 2015;61(2):107-12.
Figures