2603
System for Delayed Intervention in MRI1Imperial College Healthcare NHS Trust, London, United Kingdom, 2Bioengineering, Imperial College London, London, United Kingdom
Synopsis
A system for Interventional MRI using Infrared tracking of a handeld needle guide was adapted for practical use in closed-bore scanners. This was achieved by mapping of the image space to a patient space with the table outside the scanner bore to permit delayed visualisation of the targeted site. This novel method enables manual intervention of complex sites to be handled with greater care than other methods, and provides a relatively low-cost option for MR-guided interventions.
Introduction
Interventional procedures using MRI-guidance include biopsies and treatments such as tumour ablation1-3, typically done using open-bore scanners. The preference for closed-bore MR scanners prevents many centres from exploiting available options. A reliable method of overcoming this is required to take advantage of the increased accuracies and better outcomes possible.
To tackle closed-bore interventions, various remote-control devices have been developed, typically providing clinicans with 'end of the bore' control of a device and in-room image visualisation. A number of MRI-compatible robotic systems have also successfully been employed inside the scanner bore. Both of these relatively complicated approaches fail to provide the simple reassurance associated with manual intervention4-5.
Here we describe a third option of 'delayed' intervention by Infrared tracking of a handheld device, with real-time translation and visualisation of a 3D imageset displaying the projected path of the needle to the clinician. This system allows image-guided manual interventions to take place immediately after imaging, with the patient table outside of the bore.
This work is an ongoing part of clinical MR-guided interventions, including both laser and microwave tumour ablations, and has the ability to hugely simplify biopsy procedures.
Methods & Materials
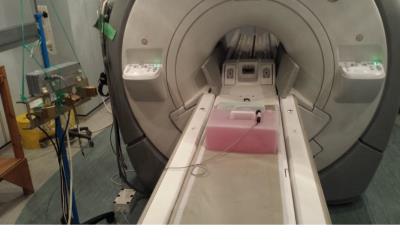
A Flashpoint 5000 Infrared (IR) tracking system was salvaged from a 0.5mT GE Signa SP/i 'double-donut' scanner; the system comprised of two light-emitting diodes (LEDs) attached to a needle guide, a 3D digitiser, a signal processor and three CCD cameras mounted on a stand (see Figure 1). This was attached to an external PC programmed with MatLab (Mathworks, USA) to read the data and collect images directly from a GE MR750 scanner (GE Healthcare, USA).
Before scanning, the CCD array -mounted horizontally on a
moveable stand, was wheeled into a suitable
position at the end of the scanner
bore. A registration process was then completed by tracking the needle
guide at four set locations around the workspace.
A LAVA sequence was used to acquire image sets of various phantoms, with the table positions recorded before bringing the table to rest outside the bore.
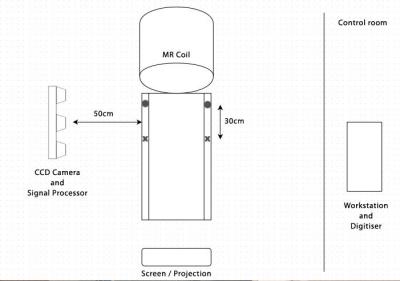
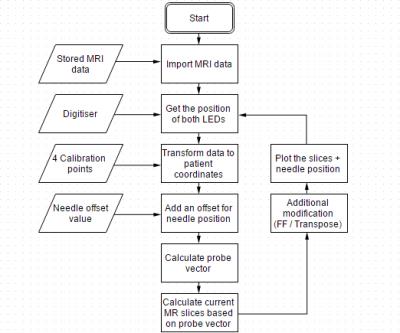
The MatLab application is then able to tranform the image data to the end-of-bore co-ordinate sytem, and combines registration data with the needle tracking values, to reconstruct images reflecting the real-time position and orientation. Finally, various images are displayed on a projector screen to guide the clinician. Figure 2 shows a diagram of the setup. The software algorithm is shown in Figure 3.
The accuracy of the system was measured by the mapping error of the transformed phantom image data for a range of needle positions and orientations across the workspace. Practical assessment of the targeting accuracy was achieved by repeated targeting of oil capsules inserted into a liver sample (see Figure 4), and comparison of the accuracy obtained without the guidance of the images.
Results
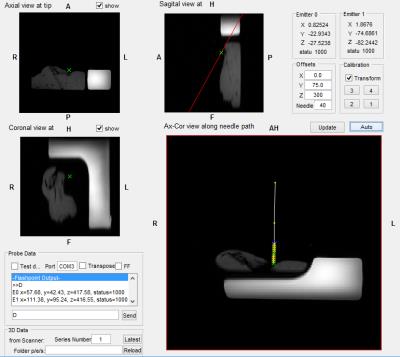
Images displayed on the external PC and through the projector allowed the user to follow the position of the needle relative to the scanned anatomy. This resulted in average errors of 2mm with a maximum of 5mm at the edge of the workspace, and displayed images with a typical update rate of up to 3fps. A screenshot is shown in Figure 5.
Handheld intervention sucess was recorded as distance from the intended target oil capsule, and had a rate of success of 95% of hitting the lesion, with an overall accuracy of 2mm. This compared favourably with a success rate of 65% and an accuracy of 12mm without the using MR images for guidance.
Discussion
The accuracy improvements measured in phantom tests show the importance of increasing the options for clinical intervention, and this is another way that this can be achieved that is flexible enough to be adapted to different procedures. Overall the accuracy is comparable with other techniques. In this application there are added problems due to accumulated errors during repeated breatholding, and further tests are needed before in-vivo work is undertaken. However it should be noted these problems also arise when using other common methods in the abdomen.
For biopsy of other anatomical sites it provides a simpler approach that can add to current techniques in prostate and breast biopsy among others.
While the Flashpoint IR system provides good accuracy and easy configuration, the system may use other similar devices or indeed tracking methods. Further work will investigate other potential applications with the aim of moving towards clinical use.
Acknowledgements
References
1. Needle positioning in interventional MRI procedure:
real time optical localisation and accordance with the roadmap. Viard,
Romain, et al. Lyon, IEEE EMBS, 2007.
2. Comparison of X-ray Fluoroscopy and Interventional MRI for the Assessment of Coronary Artery Stenoses in Swine. Green, Jordin D., et al. Chicago : PMC , 2006.
3. MRI-Guided Electrophysiology Intervention. Halperin, Henry R. and Kolandaivelu, Aravindan. s.l. : Rambam Maimonides Med, 2010.5. Ten Years’ Experience With Needle Biopsy in the Early Diagnosis of Sacroiliiti. Gong, Yao, et al. 5, s.l. : ARTHRITIS & RHEUMATISM , 2012, Vol. 64.
4. Magnetic Resonance Imaging-Guided Percutaneous Biopsy
of Musculoskeletal Lesions. Carrino, John A., et al. s.l. : Journal of
Bone and Joint Surgery, 2007.
5. Integrated active tracking detector for MRI-guided interventions. J, Anders, et al. s.l. : Mag Reson Med, 2012.
Figures