2600
Detection of temperature induced viscoelasticity changes using MR acoustic radiation force impulse imaging1Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Department of Radiology and Imaging Sciences, University of Utah, 3Department of Radiology, Stanford University
Synopsis
MR acoustic radiation force impulse (MR-ARFI) imaging, which measures tissue displacement caused by the concentrated force of a focused ultrasound (FUS) beam, has been used to locate and characterize the ultrasound focus. It has also been used to measure and assess changes in tissue elastic properties. We hypothesize that localized heating may denature elastic properties of tissues, and convert the elastic displacement caused by FUS to a form of viscous streaming. In this work, we propose using 3D GRE MR-ARFI with a unique timing scheme, to quickly and efficiently detect temperature induced viscoelasticity changes in a gelatin phantom during FUS heating.
Introduction:
MR acoustic radiation force impulse (MR-ARFI) imaging, which measures tissue displacement caused by the concentrated force of a focused ultrasound (FUS) beam, provides information about the location and shape of the ultrasound focus (1-4). Because of this property, ARFI is increasingly being used in pre-ablation planning and shows promise for guiding FUS procedures (2). Although most MR-ARFI methods use spin echo techniques and are not sensitive to temperature (5), gradient echo methods can measure displacement and temperature (6,7). MR-ARFI has previously been used to assess and detect changes in tissue elastic properties (8,9). We conjecture that localized heating may denature elastic properties of tissues, and convert the elastic displacement to a form of viscous streaming. In this work, we propose using 3D GRE MR-ARFI with a unique timing scheme to quickly and efficiently detect temperature induced viscoelasticity changes in a gelatin phantom during FUS heating.Methods:
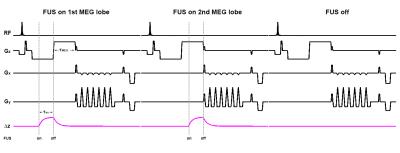
The viscoelastic MR-ARFI sequence was implemented by interleaving ultrasound pulses on the first and second motion encoding gradient (MEG) lobes of a 3D GRE MR-ARFI sequence as shown in Figure 1. To separate temperature from displacement, an FUS off image is also interleaved. If tissue is displaced elastically, displacement will occur primarily within the selected MEG lobe as shown in the figure. If the tissue is displaced with no elastic restoring force, the displacement will remain after termination of the FUS pulse, resulting in a different phase evolution depending on the lobe of placement.
Experiments were performed using a 256-element 13cm focal length transducer (Imasonic, Besançon, France) operated at 1 MHz. A single loop custom-build receive coil was used to detect the MR signal. Imaging was performed on a Siemens 3 Tesla (T) Prisma scanner (Erlangen, Germany). Each ultrasound pulse was applied for a duration of 10 ms at a power of 33 Watts. The MEG gradient amplitude was 10 mT/m with slew rate of 100 T/m/s and a duration of 16 ms. Imaging parameters included FOV 74 x 74 x 28 mm, resolution 1.16 x 1.16 x 2 mm, (zero filled interpolated to 0.58 x 0.58 x 0.5 mm voxel spacing). TR/TE = 64/46 ms, flip angle = 25°, readout bandwidth = 752 Hz/pixel, echo-spacing = 2.56 ms (with flyback), ETL = 7, giving an acquisition time of 8.96 s/image, and 26.88 s for the interleaved 3 images as shown in Figure 1. The phase change due to heating is obtained from the phase evolution of the ultrasound off image. Complex phase subtraction is used to remove this temperature phase from the ultrasound on images, leaving phase due to apparent displacement only.
Results:
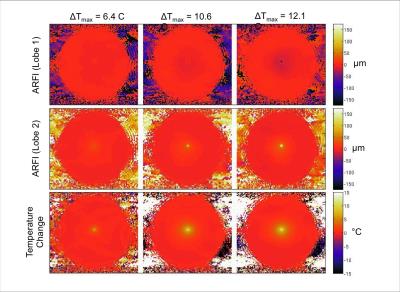
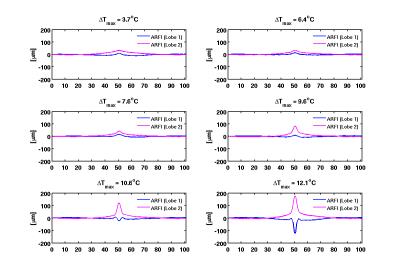
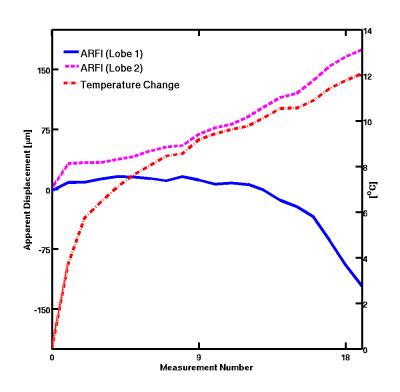
ARFI displacement maps for FUS applied during the first and second MEG lobes as well as the corresponding temperature change in degrees are depicted in Figure 2 for three different time points. At the higher temperatures (later time points) the ARFI measurements from the two lobes deviated. The deviation is shown graphically in Figure 3. Initially at lower temperatures the two ARFI measurements are in the same direction. The displacement in Lobe-1 is less than that in Lobe-2 due to the residual displacement that is still relaxing during the start of Lobe-2. Once the focal spot reaches a certain temperature, the position restoration appears to no longer occur, yielding a net negative apparent displacement. The displacement for both lobes and the temperature at the central point are plotted in Figure 4.Conclusions:
We demonstrate that temperature dependent viscoelasticity changes can be detected using novel ARFI pulse sequence timing where FUS application is interleaved between the positive and negative lobe. Although these preliminary results were shown in a phantom experiment, this protocol may prove useful in monitoring ablated volume or lesions created in real time.Acknowledgements
Funding by NIH RO1CA172787, RO1EB013433, and a University of Utah seed grant, the Focused Ultrasound Foundation, and the Mark H. Huntsman Chair.References
1. Larrat B, Pernot M, Montaldo G, Fink M, Tanter M. MR-guided adaptive focusing of ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 2010;57(8):1734-1737.
2. McDannold N, Maier SE. Magnetic resonance acoustic radiation force imaging. Med Phys 2008;35(8):3748-3758.
3. Chen J, Watkins R, Pauly KB. Optimization of encoding gradients for MR-ARFI. Magn Reson Med 2010;63(4):1050-1058.
4. Kaye EA, Chen J, Pauly KB. Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn Reson Med 2011;65(3):738-743.
5. de Bever J, Todd N, Odeen H, Parker DL. Evaluation of a 3D MR Acoustic Radiation Force Imaging Pulse Sequence Using a Novel Unbalanced Bipolar Motion Encoding Gradient. Magn Reson Med 2015;In Press.
6. Auboiroux V, Viallon M, Roland J, Hyacinthe JN, Petrusca L, Morel DR, Goget T, Terraz S, Gross P, Becker CD, Salomir R. ARFI-prepared MRgHIFU in liver: simultaneous mapping of ARFI-displacement and temperature elevation, using a fast GRE-EPI sequence. Magn Reson Med 2012;68(3):932-946.
7. de Bever J, Farrer A, Odéen H, Parker DL. Simultaneous Acquisition of MR Acoustic Radiation Force Imaging and Proton Resonance Shift Thermometry with 3D Multi-Contrast Pulse Sequence. 2014; ISMRM; Milan, Italy.
8. de Bever J, Odéen H, Parker DL. Dynamic 3D MR Acoustic Radiation Force Imaging for Tissue Property Estimation. 2015; ISMRM; Toronto, Canada.
9. Bitton RR, Kaye E, Dirbas FM, Daniel BL, Pauly KB. Toward MR-guided high intensity focused ultrasound for presurgical localization: Focused ultrasound lesions in cadaveric breast tissue. J Magn Reson Imaging 2011.
Figures