2592
Prospective Motion Correction of MR-Thermometry using an Optical Tracking System1Department Biomedical Magnetic Resonance, Magdeburg, Germany, 2Chair of Electromagnetic Compatibility, Institute of Medical Engineering, 39106, Germany, 3Institute of Neuroradiology, Magdeburg, Germany, 4Department of Radiology, Hannover Medical School, Hannover, Germany
Synopsis
Accurate temperature assessment during liver ablation requires a dedicated method for motion correction. We present the first results of a prospective motion correction method for thermometry during microwave ablation using an optical Moiré Phase Tracking system. Ex-vivo studies showed a mean temperature deviation of ΔT = 0.4 °C compared to ΔT = 34.6°C without motion correction. The method can easily be integrated into the work flow if optical tracking is applied for needle placement.
Purpose
An accurate delivery of thermal dose is directly related to a successful minimal-invasive ablation of cancerous tissue. Magnetic resonance imaging (MRI) has proven to precisely estimate temperature changes during an ablation using the proton resonant frequency shift (PRFS)1. However, in moving organs such as the liver, reliable thermometry is still a challenging task. Current motion correction (MoCo) methods for thermometry include conventional respiratory gating2, navigator echoes3, referenceless4 and multi-baseline phase correction5. We propose a simpler and potentially more accurate method for MoCo during radiofrequency (RF) or microwave ablation (MWA). Once a RF or MWA applicator is inserted, the applicator will move together with the region of interest in the liver. If the applicator is precisely tracked, prospective MoCo could be applied leading to locally accurate temperature maps even in structures moving non-rigidly. We implemented this method and present the first results in ex-vivo turkey muscle using the optical Moiré Phase Tracking (MPT) system. To our best knowledge, this is the first method enabling full six degrees of freedom (6DOF) motion correction of thermometry data.Material and Methods
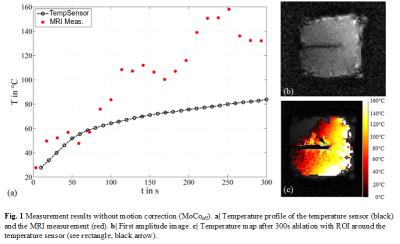
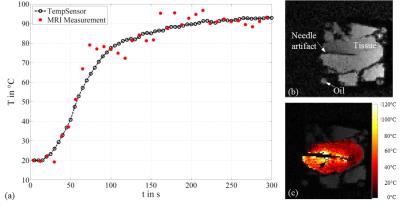
A MWA ablation needle (MedWaves, San Diego, USA) with an attached Moiré Phase (MP) marker was inserted into an ex-vivo turkey muscle. The setup was periodically moved within a 3T wide-bore MRI scanner using an in-house built drive unit. 6DOF pose data of the MP marker were continuously and accurately monitored by an MRI-compatible camera. A gradient echo sequence, capable of real-time prospective position update once per k-space line based on the real-time MP tracking data6, was adapted for PRFS-based thermometry. The parameters were the following: TE = 3.6 or 5ms, TR=50ms, voxel size = 1.9x1.9x3mm, FOV = 150x150mm, BW = 290Hz/Px, flip angle = 25°, TA = 3.8s/slice. Magnetic field drift was corrected using external oil references1. An optical temperature sensor was inserted close to the needle. Temperature maps with (MoCoon) and without motion correction (MoCooff) were acquired over an ablation period of 300s. The resulting temperature profiles were compared to the temperature sensor.Results
The maximum translational movement of the MWA ablation needle, recorded by the attached MP marker, was Δxmax=1.9mm, Δymax=8.2mm and Δzmax=7.9mm. The maximum rotational movement was Δθxmax=3.7°,Δθymax=0.3° and Δθzmax=0.9°. The results are displayed in Fig. 1 and 2. MoCoon during thermometry acquisition resulted in much more reasonable temperature distribution around the ablation needle as well as more accurate quantitative temperature measurements compared to MoCooff. The mean temperature deviation between the temperature sensor and the corresponding region of interest (ROI) in the MRI image with MoCooff and MoCoon amounted to TMoCooff=34.6°C±25°C and TMoCoon=0.4°C± 5°C, respectively. The high remaining standard deviation during MoCoon is mainly caused by residual RF-noise induced by the MWA generator.Conclusion
Motion-corrected thermometry in moving organs such as the liver is a major requirement for an accurate thermal dose assessment. The presented prospective MoCo method has shown to dramatically increase the accuracy of MR-based real-time thermometry in moving structures. If optical tracking is used to guide the positioning of the ablation needle, the method can easily be integrated into the ablation work flow.Acknowledgements
The work of this paper is funded by the Federal Ministry of Education and Research within the Forschungscampus STIMULATE under grant number '13GW0095A' and '13GW0095C'.References
1. Wyatt C, Soher B, Maccarini P, et al. Hyperthermia MRI temperature measurement. Int J Hyperthermia. 2009; 25(6):422-433.
2. Weidensteiner C, Kerioui N, Quesson B, et al. Stability of real-time MR temperature mapping in healthy and diseased human liver. J Magn Reson Imaging. 2004; 19(4):438-446.
3. De Zwart JA, Vimeux FC, Palussière J, et al. Online correction and visualization of motion during MRI-controlled hyperthermia. Magn Reson Med. 2001; 45(1):128-137.
4. Salomir R, Viallon M, Kickhefel A, et al. Reference-free PRFS MR-thermometry using near-harmonic 2-D reconstruction of the background phase. IEEE Trans Med Imaging. 2012; 31(2):287-301.
5. Vigen KK, Daniel BL, Pauly JM, et al. Triggered, navigated, ,multi-baseline method for resonance frequency temperature mapping with respiratory motion. Magn. Reson. Med. 2003; 50(5): 1003-1010.
6. Stucht D, Danishad KA, Schulze P, et al. Highest Resolution In Vivo Human Brain MRI Using Propsecitve Motion Correction. Plos One. July 30, 2015; A1. http://journals.plos.org. Accessed October 26, 2016.
Figures