2591
Fast Temperature Estimation Using Golden Angle Radial from Undersampled K-Space for MR Guided Microwave Ablation1Department of Biomedical Engineering, Tsinghua University, Beijing, People's Republic of China, 2Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, People's Republic of China, 3Department of Orthopedics, First Affiliated Hospital of PLA General Hospital, Beijing, People's Republic of China, 4Key Laboratory of Particle and Radiation Imaging, Ministry of Education, Medical Physics and Engineering Institute, Department of Engineering Physics, Tsinghua University, Beijing, People's Republic of China
Synopsis
Golden angle radial sampling is insensitive to motion and provides oversampled central k-space for self-navigation. This study aims to accelerate the acquisition with motion correction for MR temperature imaging by applying golden angle radial to hybrid model based on PRF method. The proposed method uses low resolution images obtained from a certain ratio of radius in k-space center to correct the period motion and magnetic field drift. Then, an iterative method is adopted to estimate the temperature by the hybrid model. Simulations and in-vivo experiments demonstrate the robustness to motion and effectiveness of the proposed method.
Purpose
Methods
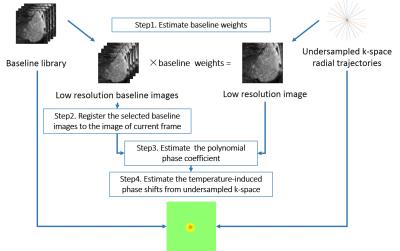
The proposed method estimates temperature changes from undersampled GA radial data based on the hybrid model[1],which could be expressed as:$$y_{i}=\sum_{j=1}^{N_{s}}e^{i\overrightarrow{k_{i}}\cdot\overrightarrow{x_{j}}}\left(\sum_{l=1}^{N_{b}}b_{l,j}{w_{l}}\right)e^{i({\left\{Ac\right\}}_j+\theta_{j})}+\epsilon_{i}$$ where $$$y_{i}$$$ is one k-space data sample, $$$N_{s}$$$ is the number of image voxels, $$$\overrightarrow{k_{i}}$$$ is the k-space location of sample $$$i$$$, $$$\left\{b_{l}\right\}_{l=1}^{Nb}$$$ are complex baseline library images which acquired before heating with different motion states, $$$w_{i}$$$ are baseline image weights, $$$A$$$is a matrix of smooth basis function, $$$c$$$ is a polynomial phase coefficient vector, $$$\theta$$$ is a heating-induced phase shift and $$$\epsilon$$$ is complex Gaussian noise[1].
As shown in Fig.1, the first step of the proposed method is the estimation of the baseline library weights to correct the period motion[1]. In this study the weights of multi-baseline images are calculated by utilizing information from low resolution images[4] reconstructed by the data with a certain ratio of radius in k-space center. The second step is using the unheated region of the low resolution images to calculate the polynomial phase coefficients for correcting the magnetic field drift[4]. Finally, the temperature estimation is conducted using an iterative method with smooth constraint.
Experimental Designs
The phantom and, in-vivo experiments with heating simulation were performed to investigate the efficiency of the proposed method. The phantom and in-vivo (liver, spine) data with large motion errors due to dynamic acquisition were acquired on a Philips 3T system (Philips Healthcare, Best, the Netherland) respectively.
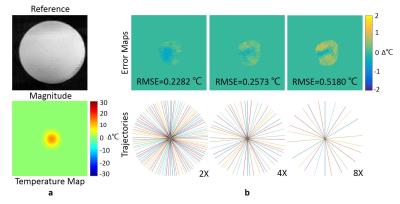
An agar phantom without heating was acquired using GA radial sequence for testing the accuracy and the computation time of the proposed algorithm. Then a Gaussian phase was added manually on the images to simulate heating (+25℃). The imaging parameters were: FOV = 160×160 mm2, in-plane resolution = 2×2 mm2, TR/TE = 50/10 ms, flip angle = 15°.
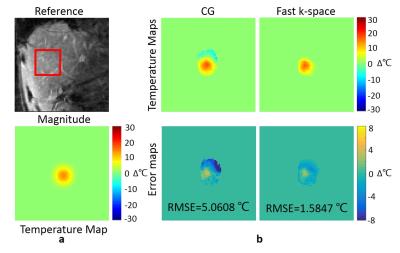
The in-vivo liver experiment with simulated heating was conducted to measure the robustness to motion and the temperature accuracy. The data was acquired using a Cartesian sequence and then converted into GA radial data using NUFFT. The scan parameters were: FOV = 240×307.5 mm2, in-plane resolution = 2×2 mm2, TR/TE = 50/10 ms, flip angle = 15°.
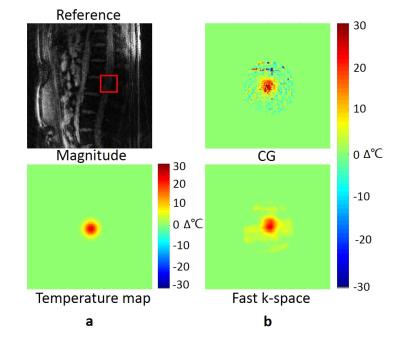
The in-vivo spine experiment without heating was performed to evaluate the performance of the proposed method for regions with complicated structures. The image was acquired using GA radial sequence on a health volunteer with following scan parameters: FOV = 256×256 mm2, in-plane resolution = 2×2 mm2, TR/TE = 30/10 ms, flip angle = 15°.
In addition, full-sampled CG temperature reconstructions were also implemented for comparison using the same sampling pattern in each experiment.
Results
In phantom heating simulations, the proposed method gave a relatively lower RMSEs and less computation time: the RMSE of the image at 4x acceleration is 0.2573℃ with computation time 6.63s, which was much shorter than the conventional hybrid model[4] (Fig.2).
In the liver experiment, the RMSE of the full-sampled CG reconstruction without motion correction is 5.0608℃ while the RMSE of the GA fast k-space reconstruction at 4x acceleration is 1.5847℃ (Fig.3).
The second in-vivo experiment was the GA sampling spine temperature reconstruction with lower SNR. When applying the radial sampling fast k-space methods under the acceleration factor of 4, the error of the temperature was much lower than the full-sampled CG reconstruction due to the motion correction and smooth constraint (Fig.4).
Discussion and Conclusion
According to the results, both phantom and in-vivo experiments validated the effectiveness of the proposed fast k-space estimation using GA radial for more accurate temperature estimation with corrected motion-induced errors. In addition, the accelerated acquisition by undersampling and relative short computation time of the proposed method make real-time temperature monitoring for MR-guided microwave ablation feasible.Acknowledgements
This work is supported by Students Research Training (SRT) research funding from Tsinghua University.References
[1] Gaur P, Grissom W A. Accelerated MRI thermometry by direct estimation of temperature from undersampled k-space data.[J]. Magnetic Resonance in Medicine, 2015, 73(5):1914–1925.
[2] Vigen K K, Daniel B L, Pauly J M, et al. Triggered, navigated, multi-baseline method for proton resonance frequency temperature mapping with respiratory motion.[J]. Magnetic Resonance in Medicine, 2003, 50(5):1003-10.
[3] Grissom W, Pauly K B, Lustig M, et al. Regularized referenceless temperature estimation in PRF-shift MR thermometry[J]. Proceedings, 2009:1235-1238.
[4] Wang F, Dong Z, Chen S, et al. Fast Temperature Estimation from Undersampled k-Space with Fully-Sampled Center for MR Guided Microwave Ablation.[J]. Magnetic Resonance Imaging, 2016, 34(8):1171-1180.
Figures