2587
Hybrid MR-ultrasound acquisition for multi-baseline thermometry1Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
MR thermometry, based on proton resonance frequency (PRF) shift, can be achieved by the phase subtraction between pretreatment baseline images and treatment images. However, breathing motion leads to phase errors that may corrupt temperature measurements. We propose the use of a hybrid MR-ultrasound imaging setup and reconstruction algorithm to provide the phase reference required for PRF thermometry in moving organs. The generated synthetic MR-ultrasound images match the acquired images in all respect except for the fact that they do not contain any heating information, and thus provide a valuable non-heated phase reference from which temperature changes can be quantified.
Introduction
The proton resonance frequency (PRF) shift method has become the prevalent technique for MR thermometry1. Breathing motion creates phase shifts that may corrupt temperature measurements, and two main approaches have been proposed to handle such motion: the referenceless2 and the multi-baseline3 approach. A hybridized version of the two has also been proposed4. The present work introduces a method for thermometry in moving organs that fits in the same general category as the multi-baseline approach. As in all such multi-baseline methods, images acquired before heating are employed to synthesize a phase reference, from which temperature-induced phase shifts can be measured. However, instead of using navigator echoes and/or a respiratory belt to characterize motion, a hybrid MR-ultrasound imaging system equivalent to that described in Ref5 was implemented here. Thermometry is typically performed for guidance purposes, during image-guided therapies, and a respiratory belt would involve much fabric and Velcro that would get in the way of an interventionalist; in contrast, the ultrasound probe employed here contacts only a small location on the torso and directly senses organ position. Furthermore, navigator echoes typically take time away from the acquisition process and are quite limited in terms of information content; in contrast, the signal from our organ configuration motion (OCM) ultrasound sensor was acquired in parallel with the MRI signal and proved very rich in terms of information content. Minimally-invasive thermal therapies in abdominal organs of wakeful free-breathing patients may become a desirable alternative to surgical resection, but robust free-breathing implementations of the PRF approach continue to be difficult to achieve. We hope that the proposed hybrid OCM-MR imaging method may prove part of the solution.Methods
Human scans were performed after informed consent, on a 3.0T Siemens Verio system (45 mT/m, 200/T/m/s), using a flexible body coil array, with parameters: FOV = 38×38 cm2, matrix size = 192×192, slice thickness = 5 mm, flip angle = 30°, bandwidth = 390 Hz/px, TR/TE = 10/4.8 ms, 500 dynamic acquisitions, temporal resolution = 0.6 s, partial Fourier ratio = 5/8. An MR-compatible, single-element OCM sensor (Imasonics, 15-mm diameter, 1 MHz) was applied to the abdomen using surgical tape. Figure 1 shows an OCM sensor, a transducer able to provide rich motion-related information as its non-focused beam may probe a wide section of the abdomen. Fiberoptic temperature probes (Neoptix ReFlex) were in contact with the OCM sensor, to detect any possible heating and prevent skin burns, but no such heating was detected. In one of two volunteers, a Hot & Cold compress was applied to the abdomen, in-between the flexible coil and the OCM sensor, to create some small but detectable temperature changes. The Bayesian learning algorithm from Ref5 was employed here to combine the streams of OCM and MRI data. Training was limited to baseline scans, about one minute worth of scans without temperature changes, such that all hybrid OCM-MR images subsequently generated by the algorithm did not feature any heating-related information, and for this reason they provided a valuable motion-matched phase references from which the PRF effect and temperature changes could be quantified, in moving organs.Results
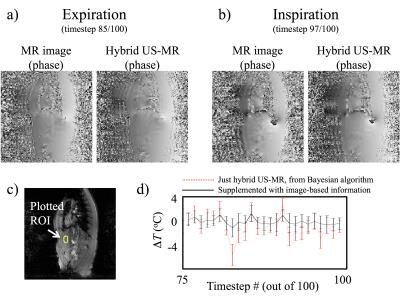
Figure 2 showed the thermometry results from a free-breathing but non-heated dataset. Both the acquired MR image and a corresponding hybrid MR-US were shown side-by-side at expiration (Fig. 2a) and at inspiration (Fig. 2b). The mean and standard deviation for temperature measurements within the ROI (Fig. 2c) were plotted from timestep theat onward (Fig. 2d). Both OCM-based and image-based information were employed toward generating the non-heated phase reference. Because there was no heating source in this acquisition, a temperature elevation of 0˚C was expected, as seen in Fig. 2d. Figure 3 demonstrated results from the second volunteer, with Hot & Cold compress. The shallow ROI1 was closer to the compress and saw temperature changes, while ROI2 was deeper and saw none (Fig. 3d, about 3˚C change for ROI1).Discussion and Conclusion
A new multi-baseline free-breathing thermometry approach was introduced, based on hybrid OCM-MR imaging. After about a minute worth of training data, the learning Bayesian algorithm could generate matching images to any new acquired MR image. These synthetic matching images were similar to acquired ones in all respect, except for the fact that they did not include any heating-related information, as only non-heated images were employed for training purposes. As a result, these hybrid OCM-MR images provided valuable phase reference maps from which temperature changes could be quantified.Acknowledgements
No acknowledgement found.References
[1] Ishihara, Y., et al., A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med, 1995. 34(6): p. 814-23.
[2] Rieke, V., et al., Referenceless PRF shift thermometry. Magnetic resonance in medicine, 2004. 51(6): p. 1223-1231.
[3] Vigen, K.K., et al., Triggered, navigated, multi-baseline method for proton resonance frequency temperature mapping with respiratory motion. Magn Reson Med, 2003. 50(5): p. 1003-10.
[4] Grissom, W.A., et al., Hybrid referenceless and multibaseline subtraction MR thermometry for monitoring thermal therapies in moving organs. Medical physics, 2010. 37(9): p. 5014-5026.
[5] Preiswerk, F., et al., Hybrid MRI-Ultrasound acquisitions, and scannerless real-time imaging. Magn Reson Med, 2016.
Figures