2585
Percutaneous MRI-Guided Cryoablation of Regional Nodal Metastases in Prostate Cancer: Initial Experience1Mayo Clinic, Rochester, MN, United States
Synopsis
In this retrospective study, we examined the feasibility and safety of magnetic resonance imaging (MRI)-guided percutaneous cryoablation of regional nodal metastases in the setting of prostate cancer. The study cohort comprised eight patients with biochemically recurrent prostate cancer who had undergone prior prostatectomy and radiotherapy, thereby limiting their ability to receive further radiation to regional nodes. Technical success was achieved in all cases and there were no procedure-related complications. Our data suggest that MRI-guided cryoablation offers a feasible alternative therapy for local control of metastatic lymph nodes. Further prospective investigation is warranted to assess short- and long-term local efficacy.
INTRODUCTION:
Magnetic resonance imaging (MRI)-guided percutaneous cryoablation is a promising treatment for native or recurrent prostate cancer (PRCA). In many patients with recurrent PRCA, metastases to regional lymph nodes occur within previously irradiated areas, thereby limiting the ability to deliver subsequent radiotherapy. To that end, we aimed to determine the feasibility and safety of MRI-guided percutaneous cryoablation of regional nodal prostate metastases.METHODS:
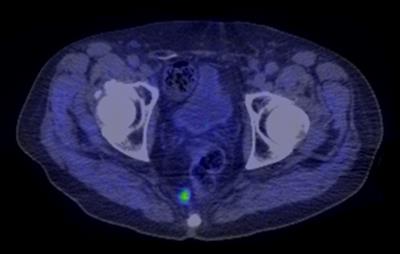
In this single-institution retrospective study, we identified patients undergoing MRI-guided cryoablation of pelvic nodal metastases in the setting of biochemically recurrent PRCA through review of an institutional database. Biochemical recurrence was determined on the basis of rising prostate serum antigen (PSA) in excess of 0.2 ng/mL. Nodal metastases were identified using multiparametric magnetic resonance imaging (mpMRI) (Fig. 1) or 11C-choline positron emission tomography/computed tomography (PET/CT) (Fig. 2). Electronic medical record and imaging review was used to identify demographic, clinical, and imaging characteristics.RESULTS:
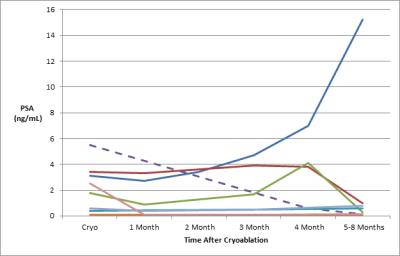
Eight patients (mean age: 63 years, range: 56-77) with biochemically recurrent PRCA were treated with MRI-guided cryoablation for regional nodal metastases. All patients had undergone prior prostatectomy for PRCA (mean Gleason score 7, range: 6-9) and 7/8 (88%) had received prior radiation therapy. The targeted perirectal lymph nodes were successfully ablated in all patients (9/9, 100%) (Fig. 3). There were no procedure-related complications. Seven patients experienced recurrent disease manifesting as a rise in PSA following the initial post-ablation nadir (Fig. 4), all within three months of ablation. Six of the recurrent cases underwent subsequent 11C-choline PET/CT which revealed choline uptake consistent with disease activity in the prostatectomy bed (n=1), regional and distant lymph nodes (n=4), and bone (n=2). Only one of the patients had imaging suggestive of residual disease around the ablation margin.DISCUSSION:
The population studied herein represents a complex subgroup of patients with metastatic nodal PRCA and a history of surgical resection and radiotherapy. Treatment options in this group are understandably limited. MRI-guided percutaneous cryoablation is an emerging technique which is not hindered by the effects of prior surgery or radiation. It benefits from excellent soft-tissue resolution and allows for continuous multi-planar visualization of the target area, permitting precise delineation of the ablation margin and thereby minimizing the potential for non-target ablation. Our initial experience demonstrates the feasibility and safety of this technique as all cases were technically successful with no procedure-related complications. While the post-ablation disease progression rate was high, most recurrences occurred outside the ablation zone and are a product of the advanced nature of metastatic disease in this high-risk cohort. This study does suggest that cryoablation does offer a good alternative therapy for local control of metastatic lymph nodes. However, this study also illustrates that this therapy cannot be used solely without systemic treatment considerations.CONCLUSION:
MRI-guided percutaneous cryoablation of regional nodal prostate metastases is safe and technically feasible. Further prospective investigation is warranted to assess short- and long-term local efficacy.Acknowledgements
No acknowledgement found.References
1. Woodrum DA, Kawashima A, Gorny KR, Mynderse LA. Targeted prostate biopsy andMR-guided therapy for prostate cancer. Abdom Radiol (NY). 2016 May;41(5):877-88.
2. Woodrum DA, Kawashima A, Karnes RJ, Davis BJ, Frank I, Engen DE, Gorny KR,Felmlee JP, Callstrom MR, Mynderse LA. Magnetic resonance imaging-guidedcryoablation of recurrent prostate cancer after radical prostatectomy: initial single institution experience. Urology. 2013 Oct;82(4):870-5.
3. Gangi A, Tsoumakidou G, Abdelli O, Buy X, de Mathelin M, Jacqmin D, Lang H.Percutaneous MR-guided cryoablation of prostate cancer: initial experience. Eur Radiol. 2012 Aug;22(8):1829-35.
Figures