2584
Functional neuroimaging using dynamic radial 3D UTE pulse sequences1Gordon Center for Medical Imaging, Massachusetts General Hospital & Harvard Medical School, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Radiology, Stony Brook University, Stony Brook, NY, United States, 4Psychiatry, Stony Brook University, Stoney Brook, NY, United States, 5Nanomedicine Science and Technology Center, Northeastern University, Boston, MA, United States
Synopsis
Functional MR neuroimaging is an essential tool for studying brain activity. Cerebral blood volume (CBV) is an important indicator of brain function, but measurements are typically qualitative or relative. Furthermore, warping and signal drift necessitate significant image pre-processing with standard EPI acquisition. In this work, we utilize a radial 3D UTE pulse sequence with optimized acquisition parameters determined from phantoms and modeling. Feasibility of dynamic UTE as a functional neuroimaging method is demonstrated in non-human primates receiving NBOH-2C-CN, a 5-HT2A receptor agonist. CBV is measured dynamically throughout the whole brain and shown to agree well with an analogous EPI experiment.
PURPOSE
The three primary physiological indicators of neural activity in fMRI are changes in cerebral blood volume (CBV), blood flow and oxygenated state of hemoglobin.To isolate the CBV-induced signal change, T2*-weighted echo-planar imaging (EPI) sequences are commonly utilized with intravascular contrast agent to overshadow contrast from changes in blood oxygenation and enhance signal from CBV changes. While EPI sequences are fast, they are prone to significant distortion artifact. Additionally, macroscopic susceptibility artifacts confound the MR signal and are difficult to separate from baseline blood oxygenation level dependent (BOLD) effects; thus results are qualitative. Ultra-short time-to-echo (UTE) sequences are insensitive to susceptibility changes and extravoxular signal dephasing. Thus, we propose to use quantitative UTE contrast-enhanced (QUTE-CE) MRI to measure CBV changes dynamically.
METHODS
QUTE-CE MRI is the combination of acquisition with an optimized 3D UTE pulse sequence and an intra-vascular contrast agent1 to render a highly quantitative signal. Ferumoxytol (Feraheme, AMAG Pharmaceuticals, Waltham, Massachusetts, USA) was used for contrast.
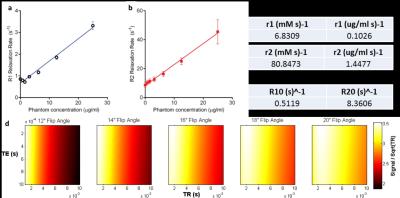
Relaxation rates were measured in 1% heparinized whole-calf-blood (Fig. 1a,-c) and modeling with the spoiled gradient echo (SPGR) equation was used to determine optimized parameters for efficient dynamic scans (Fig. 1d). CBV is calculated by simple partial volume calculations. The effect of signal modification by vascular water molecule exchange is suppressed with low TR and high FA2. Modeling signal intensity with two compartments:
$$I_M=f_BI_b+(1-f_B)I_T$$
where, $$$I_T$$$ is the brain tissue intensity, $$$I_B$$$ is the blood intensity and $$$f_B$$$ is the fraction of the voxel occupied by blood. For each image volume, CBV is calculated voxel-by-voxel by subtracting a pre-contrast image then scaling the results by the blood intensity as determined in a large vessel:
$$f_B=CBV=\frac{I^,_M-I_M}{I^,_B-I_B}$$
Animal experiments were conducted under an approved IACUC protocol. A dynamic QUTE-CE study was performed with ferumoxytol on a non-human primate (NHP) with NBOH-2C-CN, a potent and selective 5-HT2A receptor agonist, and results were compared to the current gold standard EPI + ferumoxytol imaging. All imaging was performed on a Siemens 3T Tim Trio magnet. NHPs were anesthetized throughout the scan with isoflurane (1-3%). EPI and UTE scans consisted of three imaging phases: pre-contrast followed by a bolus of 10mg/kg ferumoxytol post-contrast and then administration of 50 mcg/kg of NBOH-2C-CN. Single-shot EPI was accelerated in the phase-encode direction by a factor of two providing an isotropic spatial resolution of 1.3mm and TE=23ms.
RESULTS
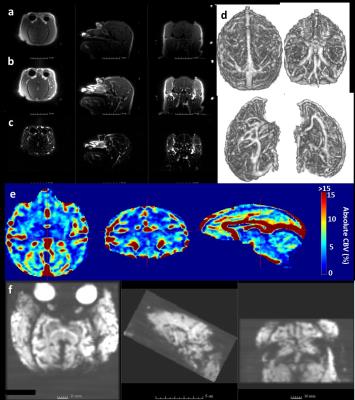
QUTE-CE pre-contrast images rendered dark blood with a small amount of tissue contrast in NHPs (Fig. 2a). Bright positive contrast of the blood was achieved after injection of ferumoxytol (Fig. 2b-d). Vasculature was clearly visible and all parts of the anatomy were free of image warping (Fig 2a-c). In contrast, time-averaged EPI data equal to the duration of the dynamic UTE scan exhibited significant signal dropout in the anterior and posterior brain, with obvious image warping (Fig 2f). The same custom-built 8-channel receive coil was used in both acquisitions. Maps of absolute CBV obtained from pre- and post-contrast UTE images are of high quality and have quantitative values consistent with expectation (Fig. 2e).
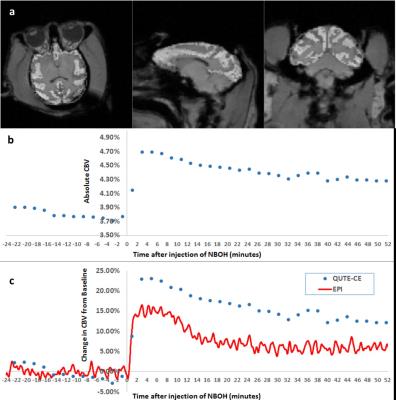
A dynamic QUTE-CE scan was performed with 1m57s time-resolution. Total scan duration was ~1.5 hours, including 48min prior to NBOH-2C-CN challenge and ~52min of data featuring drug-induced CBV changes. NBOH-2C-CN primarily affect the cortex where 5-HT2A receptors exist in high density. CBV measurements from the full cortex were compared to a separate identical experiment with EPI acquisition. Absolute CBV was measured in the cortex using the dynamic UTE method (Fig. 3b). Relative CBV changes measured by EPI and UTE exhibit strong correlation of temporal features of the response and reasonable agreement in magnitude (Fig 3c).
DISCUSSION
Sparse radial sampling is being investigated to accelerate acquisition time for each UTE volume, thereby improving the dynamic frame rate. The apparent disadvantage of a T1-based method given the high r2/r1 ratio of ferumoxytol might well be mitigated in the context of slow frequency changes, such as when study pharmacological responses3. Similar effects are presumed to occur for ASL in relation to BOLD signal4. In analogy to ASL, which has smaller signal changes than BOLD, smaller absolute signal changes in T1-based methods might find application at low frequencies due to the absolute normalization, which could remove much of the drift.CONCLUSION
To conclude, we achieved time-resolved functional imaging of brain vasculature in NHPs with a radial 3D UTE pulse sequence. Images do not exhibit spatial warping seen in EPI data and yields quantitative CBV measurements devoid of time-varying signal drift. Without baseline correction, the technique produces relative results that agree well with those obtained in a separate EPI experiment.Acknowledgements
This work was supported in part by funding from NIH R01MH100350 (MDN) and by NIH T32EB013180 (GEF).References
1. Gharagouzloo, C. A., Mcmahon, P. N. & Sridhar, S. Quantitative contrast-enhanced MRI with superparamagnetic nanoparticles using ultrashort time-to-echo pulse sequences. Magnetic Resonance in Medicine (2014). doi:10.1002/mrm.25426.
2. Kim, Y. R., Rebro, K. J. & Schmainda, K. M. Water exchange and inflow affect the accuracy of T1-GRE blood volume measurements: Implications for the evaluation of tumor angiogenesis. Magn. Reson. Med. 47, 1110–1120 (2002).
3. Jenkins, B. G. Pharmacologic magnetic resonance imaging (phMRI): Imaging drug action in the brain. NeuroImage 62, 1072–1085 (2012).
4. Wang, J. et al. Arterial spin labeling perfusion fMRI with very low task frequency. Magn. Reson. Med. 49, 796–802 (2003).
Figures