2582
Comparison of CSF Flow Imaging Methods1Radiology, Children's Hospital Los Angeles, Los Angeles, CA, United States, 2Rudi Schulte Research Institute, Santa Barbara, CA, United States, 3Neonatology, Children's Hospital Los Angeles, Los Angeles, CA, United States, 4Radiology, Children's Hospital Los Angeles, CA, United States, 5Radiology, University of Southern California, CA, United States, 6Neurosurgery, Children's Hospital Los Angeles, Los Angeles, CA, United States, 7Radiology, Children's Hospital Los Angeles, CA, 8Rudi Schule Research Institute, Santa Barbara, CA
Synopsis
We compared the quality of CSF flow images of ten subjects acquired with T2-weighted flow-void, phase-contrast, and tag-based MR methods. Tag-based methods included the variable image contrast TimeSLIP sequence and a newly designed method, termed TimeSTAMP, with constant contrast. Five radiologists and one neurosurgeon rated them on usefulness for identifying flow with a Likert scale: 5=highest to 1=lowest. Flow was detectable with high confidence for TimeSLIP and TimeSTAMP (4.8 ± 0.2), confidence was significantly lower (p<0.0001) in flow-void (2.5 ± 0.7) and phase-contrast (2.6 ± 0.5) images.
Introduction
Brain imaging permits detection and diagnosis of abnormalities such as hydrocephalus, which arises from abnormal cerebrospinal fluid production, absorption or obstruction1. There are three main MR methods for imaging CSF flow: T2-weighted flow-void2, phase-contrast3, and tag-based MR4. Flow-void and phase-contrast imaging are adversely affected by turbulent CSF flow. Tag-based flow imaging method such as Time-Spatial Labeling Inversion Pulse (TimeSLIP) are sensitive to detect turbulent and slow CSF flow, but changing background signals may render the interpretation sometimes difficult. Here we introduce a variant of tag-based flow imaging, TimeSTAMP, which minimizes the changing background signals. We acquired and compare each of these methods in healthy subjects and asked radiologists and neurosurgeons to assess them in order to compare their relative preferences.Methods
Subjects: Ten healthy subjects (5 male, 5 female, 36 ± 18 years) were recruited. Imaging
Acquisition: Studies were performed on a clinical 3T MR scanner (Titan 3T, Toshiba, Toshiba American Medical Systems, Irvine, CA).
Flow-void sensitive images were acquired with a standard clinical T2-weighted fast spin echo sequence (TE=100 ms, TR =5000 ms, FA=90 degrees, acquisition matrix=352x352, voxel resolution=0.7×0.7 (in-plane)×4.0 (thickness) mm3, sagittal phase encoding direction, flow compensation off).
For phase-contrast MRI a fast field echo sequence (TE=10 ms, TR=30 ms, FA=15 degrees, acquisition matrix=256x256, resolution=0.6×0.6×4.0 mm3, velocity encoding=10 cm/sec, 16 cardiac phases) was used.
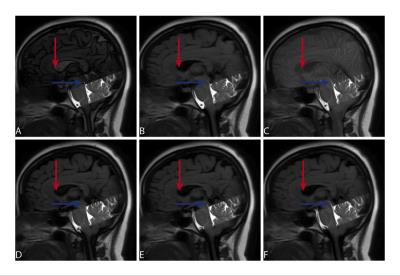
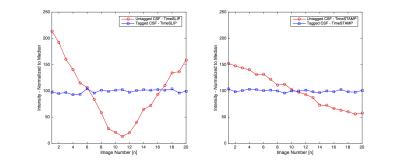
Both tagging-based methods used half-fourier fast spin echo sequence (TE=80 ms, TR=13450 ms, FA=90 degrees, acquisition matrix 224x224, voxel resolution=1.0×1.0 mm × 5.0 mm3). For TimeSLIP, twenty cardiac-gated images were acquired at delay times between initial inversion and selective tagging of 1700, 1800, … 3700 ms. For TimeSTAMP the cardiac gating was omitted and 20 images were acquired at random times points within the cardiac cycle at a quasi-constant (see legend, Figure 3) delay time of 2700ms.
Image Review: All images were reviewed by four radiologists and one neurosurgeon who rated their confidence for identifying flow for each image using a five-level Likert scale (5=best…1=worst). A two-way analysis of variance was performed using GraphPad Prism (GraphPad, La Jolla) to test for differences.
Results
Examples of each modality are presented in Figures 1 & 2. Flow was detectable with high confidence for both TimeSLIP and TimeSTAMP (4.8 ± 0.2). Confidence was significantly lower (p<0.0001) in both flow-void (2.5 ± 0.7) and phase-contrast MRI (2.6 ± 0.5 ). The TimeSTAMP sequence provided a visualization of flowing CSF (Figure 2), yet eliminated the variation of the background signal intensity (Figure 2, Figure 3).Discussion
Flow-void imaging requires no additional scan time as the needed fast spin echo sequences are ubiquitous in clinical protocols, yet they are typically acquired with flow-compensated anatomic sequences designed to reduce the prominence of flow-void. Phase-contrast MR provides quantitative flow throughout the cardiac cycle with multiple images, but lack anatomical detail, and with flow velocity unknown a-prior the VENC may reduce image quality. Tag-based imaging provides multiple images throughout the cardiac cycle and has anatomical details similar to T2-weighted images. While there was no preference of TimeSTAMP over TimeSLIP observed in this limited study in volunteers, we speculate that TimeSTAMP will be more robust in clinical settings. When the presence/absence of flow is not a priori predictable due to the unambiguous contrast difference between flowing and stationary CSF, it may provide a critical method improvement. A study to investigate this hypothesis in patients has commenced at our institution.Conclusion
In this study, tag-based images were preferred over T2-weighted flow void and phase-contrast images in our study. These new CSF imaging approaches may provide more robust diagnoses of CSF abnormalities and result in better information for management of patients with suspected hydrocephalous.Acknowledgements
No acknowledgement found.References
[1] A. Dinçer and M. M. Özek, “Radiologic evaluation of pediatric hydrocephalus,” Childs Nerv Syst, vol. 27, no. 10, pp. 1543–1562, Sep. 2011.
[2] W. G. Bradley, “CSF Flow in the Brain in the Context of Normal Pressure Hydrocephalus,” American Journal Of Neuroradiology, vol. 36, no. 5, pp. 831–838, May 2015.
[3] S. Stoquart-El Sankari, P. Lehmann, C. Gondry-Jouet, A. Fichten, O. Godefroy, M. E. Meyer, and O. Baledent, “Phase-Contrast MR Imaging Support for the Diagnosis of Aqueductal Stenosis,” American Journal Of Neuroradiology, vol. 30, no. 1, pp. 209–214, Aug. 2008.
[4] S. Yamada, K. Tsuchiya, W. G. Bradley, M. Law, M. L. Winkler, M. T. Borzage, M. Miyazaki, E. J. Kelly, and J. G. McComb, “Current and Emerging MR Imaging Techniques for the Diagnosis and Management of CSF Flow Disorders: A Review of Phase-Contrast and Time-Spatial Labeling Inversion Pulse,” American Journal Of Neuroradiology, Jul. 2014.
Figures