2576
MR Imaging of tissue near aneurysm clips using short- and zero echo time MR sequences1Biological Resources Imaging Laboratory, UNSW, Australia, Mark Wainwright Analytical Centre, Sydney, NSW, Australia, 2Central European Institute of Technology, Masaryk University, Brno, Czech Republic, 3Department of Neurosurgery, University of Vienna, Vienna, Austria, 4Department of Oncology, Division of Medical Physics, University of Alberta, Edmonton, AB, Canada
Synopsis
Aneurysm clips
are used to stop or prevent an aneurysm from bleeding. MRI is an ideal
technique to diagnose aneurysms. Unfortunately, treatment assessment by MRI
after surgical placement of an aneurysm clip is complicated due to the presence of
the metal clip. The clip's high magnetic susceptibility causes severe,
orientation dependent, variations in the local magnetic field. Often this
results in pronounced MR image distortions including signal voids. The study
presented here shows how ultra-short and zero echo time experiments could be
used to minimize these artifacts.
Purpose
An estimated 6 million people in the USA have an unruptured brain aneurysm (approximately 1 in 50). Annually about 30,000 people in the USA suffer a brain aneurysm rupture. Aneurysm clips are used to stop or prevent an aneurysm from bleeding. Unfortunately, treatment assessment by MRI after surgical placement of an aneurysm clip is problematic due to the presence of the metal clip. The clip's high magnetic susceptibility causes severe, orientation dependent, variations in the local magnetic field and strong interaction with the proton magnetization during MRI. Often this results in pronounced MR image distortions including signal voids. These effects have been studied in detail [1] but the solutions provided are limited. To reduce susceptibility artifacts several MRI techniques were adapted, using a spin- (SE) instead of gradient echo (GE), increasing the bandwidth and reducing the echo time. The artifacts could be reduced using specific sequences such as SEMAC, MAVRIC and VAT[2-4], however, they cannot completely be removed. In order to minimize the effects of the aneurysm clip on the MR image, the orientation dependent [5] perturbation of the Zeeman interaction caused by the susceptibility effect needs to be removed or minimized. Assuming the clip, in a first approximation, to be a small cylinder with a radius a, the introduced field inhomogeneity for $$$r>a$$$ is given by: $$$\Delta B(r) =\frac{\Delta \chi \cdot B_0}{2} \cdot\frac{a^2}{r^2}\{\sin^2(\theta)\cdot \cos(2\phi)\}$$$. When parallel to B0 ($$$\theta$$$ = 0) the effect is absent outside the cylinder. The perturbation is linear in B0, and thus worse at higher field strengths of 9.4T used here, while it scales with $$$\frac{1}{r^2}$$$ for distance. As a result, slice selection and MR sequences using echo times will be affected by the susceptibility effect. In this study we test the artifact reduction efficiency of short 3D (UTE) and Zero (ZTE) echo time MR sequences. Both sequences avoid slice selection and use a minimal delay after RF excitation before signal acquisition. Recently the ZTE sequence was tested on a clinical MR system [6].Methods
All experiments were performed using plastic sample vials, one containing a titanium aneurysm clip (Yasargil, Aesculap, Tuttlingen, Germany) suspended in agar gel (2% in water)and the other just agar (control). MR experiments were performed with a 9.4T Bruker Biospec, Avance III 94/20 (Bruker, Ettlingen, Germany), using a 72 mm (i.d.) birdcage quadrature RF coil. T2 and T2* relaxation times were measured on the control phantom using a MSME and MGE pulse sequence, respectively. For the vial containing the clip, MSME and MGE experiments were performed using a 8x8cm2 FOV for a 410x410 data matrix, TR= 100ms/1000ms (gradient and spin echo resp.) and a 50kHz bandwidth. UTE and ZTE experiments were performed with a 2ms RF block pulse with a 640kHz excitation bandwidth and a 256x256x256 data matrix for a FOV of 6x6x6cm3 using various acquisition parameters listed in the results section.Results and Discussion
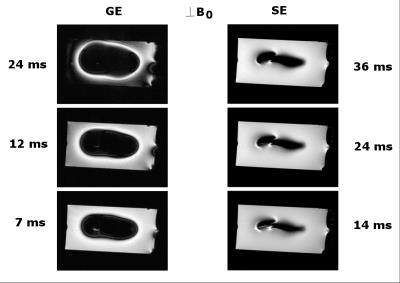
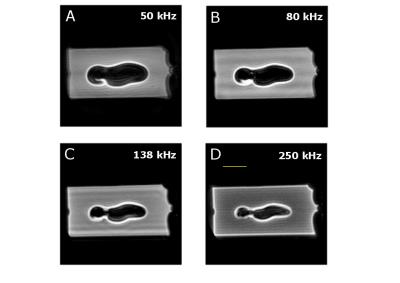
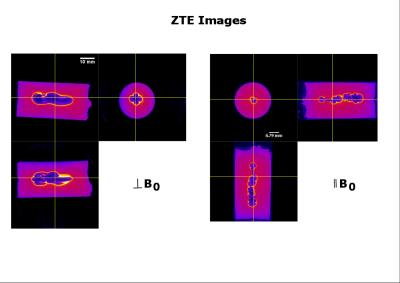
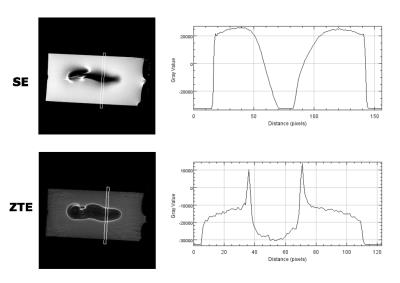
As the susceptibility of titanium ($$$\chi$$$=182 ppm)[5] is much smaller than of stainless steel or cobalt-chromium ($$$\chi$$$=4000 and 900 ppm, resp.)[5], using titanium for aneurysm clips would be preferable. Relaxation times of the control phantom were T2=50.4+/-0.8ms and T2*= 42+/-1.3ms, as an average, measured at various positions along the long axis of the vial. Figure 1 shows the susceptibility effects on standard SE and GE experiments when the vial was placed perpendicular to B0 ($$$\theta$$$=90o). The effect of time evolution during signal acquisition is shown for a selected slice in 3D UTE experiments in Figure 2. Experimental parameters: TE = 16ms, 2ms block RF pulse, TR=5ms, FOV=6x6x6cm3, 128x128x128 data points and 4.7o flip angle for 33466 projections. The acquisition bandwidth was varied from 50-250kHz. The effects of susceptibility are seen to minimize with increasing digitizer speed. Figure 3 shows that even for ZTE imaging, the effects of susceptibility cannot completely be removed. However, as shown in Figure 4, ZTE images show reduced artifact effect as compared to conventional SE experiments. The distortion of the image has a larger spread in the SE compared to ZTE, 8mm compared to 14mm for the orientation in Figure 4. As for the 3D-UTE experiments, larger acquisition bandwidths in ZTE experiments, result in smaller artifacts due to the reduction of the total acquisition time. To minimize the effects of the artifact the acquisition time should be kept to a minimum. This is only possible if one would resort to single point imaging (SPI).Conclusion
The experimental data show that 3D UTE and ZTE experiments can provide more reliable image data given the significant reduction in time evolution during signal acquisition.Acknowledgements
We would like to thank Dr. Tzong Tyng Hung and Brendan Lee of the Mark Wainwright Analytical Centre, UNSW Australia, for their help with the experiments.References
1. F. Khursheed et al. Artifact quantification and tractography from 3T MRI after placement of aneurysm clips in subarachnoid hemorrhage patients. BMC Medical Imaging 2011; 11:19
2. W. Lu et al. SEMAC: Slice encoding for metal artifact correction in MRI. Magn. Reson. Med. 2009; 62: 66-76.
3. K.M. Koch et al. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn. Reson. Med. 2009; 61, 381-390.
4. Z.H. Cho et al. Total inhomogeneity correction including chemical shifts and susceptibility view angle tilting Med Phys. 1988; 15; 7.
5. J.F. Schenk . The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996; 23: 815-850.
6. M. Weiger et al. ZTE Imaging in Humans. Magn. Reson. Med. 2013; 70: 328.
Figures