2556
T1 Black Hole in Multiple Sclerosis: a reassessment of the specificity at 7T1Vanderbilt University, Nashville, TN, United States, 2Medical University Vienna
Synopsis
7T imaging and the commensurate T1-lenghtening have the potential to increase lesion sensitivity at the expense of their specificity in patients with multiple sclerosis. In our work we demonstrate that (1) T1-hypontense lesions detected on T1-w MPRAGE reflect both remyelinated and chronic demyelinated lesions as measured by histology and that (2) associations between black hole lesion load and measures of physical and cognitive disability are weak. At 7T, the increased sensitivity to lesion penalizes the T1-w MPRAGE specificity to areas with higher water content and tissue destruction seen at lower field strength.
Purpose
In patients with multiple sclerosis (MS), the advent of T1-weighted MPRAGE sequences at 7T and the commensurate T1-lenghtening have the potential to challenge the well-recognized pathological specificity of chronic black holes (cBHs) and their ability to serve as biomarkers of more aggressive disease1. To characterize the pathological and clinical features of cBHs at 7T, we conducted this combined post mortem and in vivo study. Our hypothesis is that due to T1 increase secondary to high field imaging, cBH sensitivity will increase. As result their specificity to areas with higher water content and tissue destruction will decrease. Such a decrease in pathological specificity will ultimately make cBHs at 7T no longer surrogate markers of more advanced MS disease. To prove our hypothesis we first investigated the ability of cBHs detected on T1-w MPRAGE at 7T MRI to segregate demyelinated vs remyelinated lesions, post mortem. Second, we investigated the association between cBHs load and clinical metrics of physical and cognitive disability. Third, the heterogeneity of myelin content of cBHs in vivo was measured using quantitative magnetization transfer (qMT) imaging derived indices such as the pool sizeMethods
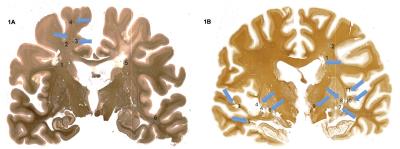
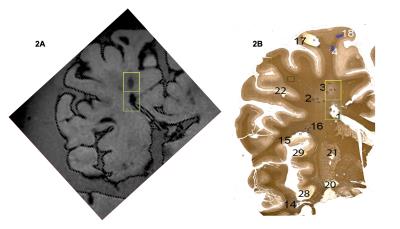
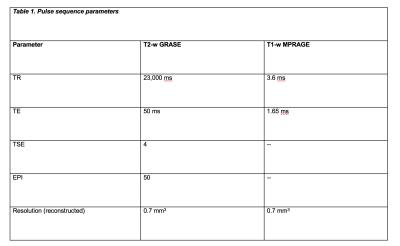
· Post mortem study All data were obtained on a Philips Achieva 7T MR unit with dual channel transmit and 32 channel head coil for reception. Seven coronal brain slices derived from brains of patients with secondary progressive (SP) MS and one with primary progressive (PP) MS were imaged using high resolution isotropic 0.7 mm3 T1 MPRAGE and T2-weighted gradient-echo spin echo (GRASE) MRIs at 7T (Table 1). On these images, cBHs were defined as areas of hypo-intense signal on T1 MPRAGE corresponding to areas of hyperintense signal on T2 GRASE images. Proteolipid protein (PLP) myelin staining was obtained for each brain sample. Shadow and chronic inactive lesions according to the definition of Prineas and collaborators2 were identified. MRI-histology side-by-side comparisons were performed to assess the correspondence between the presence of a cBH by MRI and that of a chronic demyelinated lesion by PLP. · In vivo study After signed informed consent, 25 MS patients underwent a 7T MRI using T1 MPRAGE and selective inversion recovery (SIR) qMT as well as clinical exam to rate disability with Expanded Disability Status Scale (EDSS) score3 and a cognitive evaluation using the cognitive tests listed in the Table 2. Patient cohort included 5 men and 20 women. Mean±standard deviations were 34.8±4.1 for age and 7.8±5.4 for years of MS. EDSS ranged between 0 and 6.0. cBHs were identified and marked on T1 MPRAGE and lesion load computed using MIPAV.Results
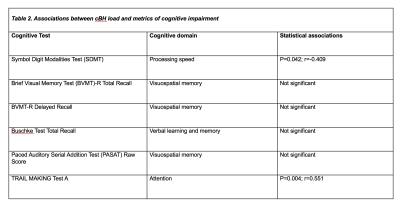
By histology (Figure 1), 51 white matter lesions were identified. Of those, 19 were shadow plaques and 32 were chronic inactive plaques. T1 MPRAGE sequences captured 52.6% shadow plaques and 71.8% chronic inactive lesions (Figure 2). In patients, there was a weak association between physical disability metrics and lesion load. Specifically, only about 23% of the EDSS variance was explained by cBH load (p=0.052, r=0.479 by Spearman correlation analysis). Table 2 shows that the associations between cBH load and metrics of cognitive impairment were of similar degree and present only for a minority of the performed cognitive tests.Discussion and conclusions
The presented results favor our hypothesis that T1 MPRAGE at 7T is sensitive to cBH detection but its pathological specificity is limited. Post mortem findings indicate that T1-weighted images, although with different degree of signal changes, capture both remyelinated and chronic demyelinated lesions, being lesion size the most important factor in determining lesion visibility. In vivo, the weak associations between lesion load at 7T and metrics of physical and cognitive disability discourage the use of cBHs as biomarker of disease severity at 7T. We hypothesize that the heterogeneous degree of myelin content subtending cBHs visible on T1 MPRAGE at 7T is responsible for loss of their pathological specificity. Results derived from qMT will be presented and provide evidence as to if our hypothesis holds true. Cumulatively the findings will help clinicians aiming at assessing the degree of disease in vivo when using ultra high filed MRI as well as scientists planning the next generation of clinical trials assessing neurodegeneration and repair using ultra-high field MRI.Acknowledgements
The authors would like to acknowledge all of the patients who volunteered for the study, Dr. Paul Newhouse for help with design of cognitive testing methods, and MRI technologists: Kristen George-Durrett, Leslie McIntosh, Clair Jones, and Chris Thompson. This work was supported in part by funding from DoD W81XWH-13-1-0073, NIH/NINDS R21NS087465, National MS Society, and NIH R01 EY023240 (PI: Smith). Tissue samples and associated clinical and neuropathological data were supplied by the Multiple Sclerosis Society Tissue Bank, funded by the Multiple Sclerosis Society of Great Britain and Northern Ireland, registered charity 207495 as well as the Rocky Mountain MS Center Tissue bank supported by the National MS Society.References
1) Barkhof F, Bruck W, De Groot CJ, et al. Remyelinated lesions in multiple sclerosis: magnetic resonance image appearance. Arch Neurol 2003; 60: 1073-1781.
2) Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001; 50: 646–57.
3) Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS).Neurology 1983; 33: 1444-145
Figures