2553
Real Time MRI-guided convection-enhanced delivery in porcine brain to model multiple sclerosis by focal demyelinationLukasz Kalkowski1, Izabela Malysz-Cymborska1, Dominika Golubczyk1, Miroslaw Janowski2,3,4, Piotr Holak5, Kamila Milewska1, Zbigniew Adamiak5, Joanna Wojtkiewicz6, Wojciech Maksymowicz1, Dorota Kedziorek2,3, and Piotr Walczak1,2,3
1Department of Neurology and Neurosurgery, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland, 2Cellular Imaging Section and Vascular Biology Program, Institute for Cell Engineering, John Hopkins University, Baltimore, MD, United States, 3Russell H. Morgan Department of Radiology and Radiological Science, John Hopkins University, Baltimore, MD, United States, 4NeuroRepair Department, Mossakowski Medical Research Center, Polish Academy of Sciences, Warsaw, Poland, 5Department of Surgery and Roentgenology with the Clinic, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland, 6Department of Pathophysiology, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland
Synopsis
Modeling of multiple sclerosis is typically performed in rodents; however, due to several limitations large animal models are needed to improve clinical relevance. In this study we utilized MRI-guided convection enhanced delivery of gliotoxins (ethidium bromide and lysolecithin) to induce focal demyelination within corona radiata in pigs.
Introduction
Multiple sclerosis (MS) is one of the most widespread neurological disorders with no effective therapy. The pathological features of the lesion include focal demyelination, axonal degeneration and inflammation as well as local astrocytosis and formation of astrocytic scar (Lucchinetti et al. 2005). Various animal models of MS had been developed, including those based on inducing autoimmune reaction against myelin epitopes, viral-induced encephalomyelitis and toxin-dependent focal demyelination (Rivers et al. 1933; Theiler 1934; Walczak et al. 2011). Vast majority of preclinical research on MS is based on rodent models which are very useful and cost effective. On the other hand, more clinically relevant models are needed to validate therapeutic strategies prior to clinical translation. Domestic pig (Sus scrofa domestica L.) has a brain that is gyrencephalic, only 10 times smaller compared to human (mouse is 1000 times smaller) and white matter content is similar to human brain. Convection-enhanced delivery (CED) is a technique which uses a convection with bulk flow of interstitial fluid into the brain parenchyma (Bobo et al. 1994). CED has been performed with various biochemical compounds in various preclinical models (Bankiewicz et al. 2000; Bobo et al. 1994) as well as in brain tumor patients (Ding et al. 2010). The low velocity of injection in CED minimizes potential damage caused by brain tissue displacement by the infused solution (Nduom et al. 2012) and results in uniform distribution of active ingredients during intraparenchymal injections (Kantorovich et al. 2013). Accordingly, the main goal of this study was to establish a model of focal demyelination in domestic pig brain with precise lesion placement using ClearPoint® system. Moreover, gliotoxin distribution was monitored in real-time- convection-enhanced delivery, based on gadolinium contrast.Materials and methods
The animal procedures were performed according to ARRIVE guidelines. Eight juvenile female pigs of 25-30 kg were anesthetized with propofol (3-5mg/kg/h i.v.) and sevoflurane (0.5-1%) and following head skin incision, a burr hole was placed. The SmartFrame® and trajectory device (MRI Interventions, Inc.) was fixed to the skull using titanium screws (Fig. 1A). Gliotoxin (2-3% lysolecithin or 0.0125-0.2 mg/ml ethidium bromide in PBS) was supplemented with gadoteridol (ProHance, 2 mM). MRI compatible SmartFlow® catheter was filled with gliotoxin, injection needle was inserted beneath the dura mater and animals were placed in a 3 T MRI scanner (Magnetom Trio, Siemens). HASTE sequence was used to adjust injection trajectory to target corona radiata. After targeting, solution was infused at 250 µl/h rate over one hour with repeated T1 scans to monitor the CED. MRI follow up was performed one day and one week post injection. MRI protocol included T2 (TR/TE= 6440/83 ms) and T1 (TR/TE= 1900/2.5 ms) with and without i.v. contrast. After last imaging session, animals were transcardially perfused with 10% sucrose, followed by 4% PFA in PBS (pH= 7.4). Brains were harvested, post-fixed, cryoprotected and frozen on dry ice. Tissue specimens were used for histological analysis including eriochrome cyanine R for myelin.Results
ClearPoint system facilitated convenient adjusting of injection trajectory under real-time MRI to navigate the needle to selected target. CED of gliotoxin/gadolinium was successfully visualized on T1w scans acquired during injection. Fig.1B shows the brain before injection and Figs. 1B1-B4 are from boxed area in B, acquired after 15, 30, 45 and 60 min show gradual expansion of the hyperintense region. MRI at one week show small region of blood-brain barrier disruption on T1+Gd scan (Fig. 1C, green arrowhead) and lesion appears as hyperintense on T2w MRI (Fig. 1D). Histological staining for myelin (Eriochrome; Fig.1E) confirmed localization of demyelination, which spatially matched with gliotoxin distribution detected on dynamic T1w and one week T2w weighted MRI (red arrowheads). Correlational analysis revealed strong correspondence between the lesion size on T2w MRI and histopathology.Conclusion
Interventional MRI-guidance was instrumental for precise induction of focal demyelination in pig. MRI and histopathological features of lesions resemble those observed in MS.Acknowledgements
No acknowledgement found.References
Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. 2000. Convection-Enhanced Delivery of AAV Vector in Parkinsonian Monkeys; In Vivo Detection of Gene Expression and Restoration of Dopaminergic Function Using Pro-drug Approach. Experimental Neurology 164:2-14.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. 1994. Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences 91:2076-2080.
Ding D, Kanaly CW, Bigner DD, Cummings TJ, Herndon JE, 2nd, Pastan I, Raghavan R, Sampson JH. 2010. Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1-1. J Neurooncol 98:1-7.
Kantorovich S, Astary GW, King MA, Mareci TH, Sarntinoranont M, Carney PR. 2013. Influence of neuropathology on convection-enhanced delivery in the rat hippocampus. PLoS One 8:e80606.
Lucchinetti CF, Parisi J, Bruck W. 2005. The pathology of multiple sclerosis. Neurol Clin 23:77-105, vi.
Nduom EK, Walbridge S, Lonser RR. 2012. Comparison of pulsed versus continuous convective flow for central nervous system tissue perfusion: laboratory investigation. J Neurosurg 117:1150-4.
Rivers TM, Sprunt DH, Berry GP. 1933. Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. The Journal of Experimental Medicine 58:39-53.
Theiler M. 1934. Spontaneous encephalomyelitis of mice- a new virus disease. Science 80:122-122.
Walczak P, All AH, Rumpal N, Gorelik M, Kim H, Maybhate A, Agrawal G, Campanelli JT, Gilad AA, Kerr DA and others. 2011. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia 59:499-510.
Figures
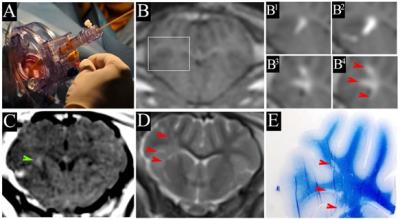
Picture of ClearPoint device mounted on porcine head (1A). 1B shows T1-weighted coronal scan before infusion of
gliotoxin/gadolinium and 1B1-1B4 show boxed area in B
after 15, 30, 45 and 60min of infusion. 1C is a gadolinium enhanced T1 scan
showing BBB disruption. 1D shows
hyperintense lesion on T2w scan seven days post injection. 1E Coronal
section of the brain lesioned with 3% lysolecithin stained for Eriochrome. Red
arrowheads shown lesion area.