2547
STRUCTURAL AND FUNCTIONAL MRI PREDICTORS OF DISABILITY AND COGNITIVE IMPAIRMENT ACCRUAL IN PATIENTS WITH MULTIPLE SCLEROSISPaola Valsasina1, Maria Assunta Rocca1, Fiammetta Pirro1, Elisabetta Pagani1, Alessandro Meani1, Massimiliano Copetti2, Filippo Martinelli Boneschi3, Vittorio Martinelli3, Giancarlo Comi3, Andrea Falini4, and Massimo Filippi1
1Neuroimaging Research Unit, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, 2IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy, 3Department of Neurology, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy, 4Department of Neuroradiology, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy
Synopsis
Aim of this study was to identify the MRI predictors of medium-term disability and cognitive impairment accrual in patients with the main clinical phenotypes of multiple sclerosis (MS). Results indicated that clinical disability and cognitive impairment at follow-up were predicted by measures of structural and microstructural damage, as well as by resting state functional connectivity measures. Preserved white matter integrity predicted clinical improvement. Grey matter involvement played a critical role in MS-related clinical worsening and evolution to a more severe disease phenotype.
Purpose
Conventional MRI (cMRI) measures have a prognostic role in patients with a clinically isolated syndrome [1], while their role in patients with definite multiple sclerosis (MS) is still debated, with recent evidences suggesting that grey matter atrophy is better associated with long term disability and cognitive impairment than focal white matter lesions [2]. Aim of this study was to assess the value of conventional MRI, diffusion tensor imaging (DTI) and resting state (RS) functional connectivity (FC) MRI measures in predicting clinical deterioration and cognitive impairment over a four-year follow-up (FU) in patients with MS.Methods
Using a 3T Philips scanner, dual-echo, 3D T1-weighted, DT and RS functional MRI scans were obtained at baseline from 248 right-handed MS patients and 98 matched healthy controls. Patients underwent a neurologic and neuropsychological evaluation at baseline and after a median period of 3.8 years of FU. Based on Expanded Disability Status Scale score [3] modifications at follow-up, MS patients were classified as clinically stable, worsened or improved. Patients were defined as cognitively worsened if the number of cognitive tests failed was greater than at baseline. Average fractional anisotropy (FA) and mean diffusivity (MD) values from the 48 white matter regions of the ICBM-DTI-81 white-matter label atlas [4] were obtained. The main sensory, motor and cognitive RS FC networks were identified using independent component analysis [5] with calculation of global and regional differences. Multivariate logistic predictive models were built using clinical worsening, evolution to a more severe clinical phenotype, clinical improvement and cognitive deterioration as dependent variables.Results
At follow-up, 35% of the patients had clinically worsened, 6% had clinically improved and 27% had cognitively worsened. Patients divided according to follow-up clinical or cognitive status, showed several statistically significant differences in lesional and atrophy measures, DT MRI and RS FC measures at baseline. Figure 1 shows the results of Random Forest analysis, which detected the ten most important MRI variables correlated with each clinical outcome. The multivariate analysis showed that lower grey matter volume and lower default mode network RS FC best predicted clinical worsening (C-index=0.67) and evolution to a more severe clinical phenotype. Higher thalamic network RS FC predicted a worsened clinical and cognitive status, probably indicating a maladaptive response. A higher fractional anisotropy in the normal-appearing white matter predicted clinical improvement (C-index=0.71). Both microstructural damage in the corpus callosum and lower executive control network RS FC predicted cognitive deterioration (C-index=0.84).Conclusions
Advanced MRI techniques are an essential tool to improve our understanding of the extreme variability in MS and allow prognosis prediction at an individual level.Acknowledgements
This study was partially supported by a grant from FISM 2014/R/7.References
[1] Giorgio A., et al., Neurology 2013; 80:234-41. [2] Filippi M., et al. Neurology 2013; 81:1759-67. [3] Kurtzke J.F. Neurology 1983; 33:1444-52. [4] Mori S., et al. Neuroimage 2008; 40:570-82. [5] Calhoun V., et al. Hum Brain Mapp 2001; 14:140-151.Figures
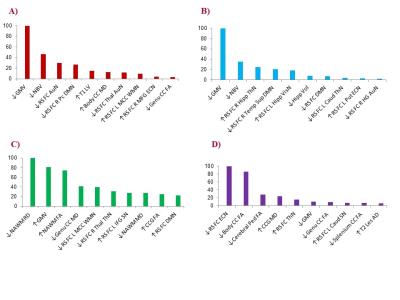
Results of
the Random Forest analysis. Normalized variable importance, ranging from 0 (not
important) to 100 (the most important), of the ten most important MRI variables
in predicting EDSS worsening (A), evolution to a more severe clinical phenotype
(B), EDSS improvement (C) and cognitive deterioration (D).