2535
Automated evaluation of deep gray matter neuronal damage in multiple sclerosis patients1Advanced Clinical Imaging Technology, Siemens Healthcare HC CEMEA SUI DI PI, Lausanne, Switzerland, 2Department of Radiology, CHUV, Lausanne, Switzerland, 3LTS5, EPFL, Lausanne, Switzerland, 4Siemens Healthcare S.A.S., Saint-Denis, France, 5Department of Neurology, University Hospital Henri Mondor, Créteil, Switzerland, 6Department of Neuroradiology, University Hospital Henri Mondor, Créteil, France
Synopsis
We investigate the potential of a technique to automatize quantification of neuronal damage from T1-weighted MR scans in multiple sclerosis patients. T1 hypointense component measures in the deep nuclei are derived from 40 MPRAGE scans (21 relapsing-remitting MS and 19 age-matched controls) through combined brain tissue classification and atlas-based segmentation algorithms. Our analysis shows that these automated measures are significantly lower in the thalamus and putamen of MS patients, which is in line with previously reported loss of structure in these regions.
Purpose
While multiple sclerosis (MS) is generally considered a primary inflammatory disease, the concept of associated neurodegeneration in MS is now widely accepted [1]. On the basis of histological studies, the extent of neuronal loss seems to be higher in the deep gray matter (DGM) than in the cortex of MS patients [2]. As DGM nuclei are composed of neurons and glial cells (exhibited as the T1 hypointense component in T1-weighted images) and neuronal fibers (T1 hyperintense component), we assume that neuronal loss will result in a proportionally lower ratio of the T1 hypointense component. This assumption is endorsed by a recent study revealing significant increase in quantitative T1 values in the gray matter component of both thalamus and putamen when comparing healthy controls to RR-MS [3]. By using a tissue classification combined with an atlas-based region segmentation algorithm, it is possible to automate the evaluation of the hypointense component in DGM structures from a conventional 3D T1-weighted scan. Our purpose is to explore whether this technique could be relevant for automated quantification of neuronal damage in MS patients.Material and methods
21 RR-MS patients and 19 age-matched normal volunteers underwent a 3D magnetization-prepared rapid gradient echo acquisition (MPRAGE) performed on a 3T MR scanner (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany): TR/TI: 2300/900 ms, voxel-size: 1.0x1.0x1.2 mm3, iPAT=2, acquisition time: 5:12 min, and a 2D FLAIR acquisition (TR/TI/TE= 9000/2500/95 ms, voxel size 1x1 mm², slice thickness: 2.5 mm). All scans were performed with a commercial 32-channel head coil.
We used the MorphoBox prototype [4] to segment and estimated the volumes of the following DGM nuclei: thalamus, caudate, putamen and pallidum. Brain tissue classification was performed on a skull-stripped brain by means of a template-free algorithm fitting a 5-class Gaussian mixture model roughly representing ventricular CSF, sulcal CSF, cortical GM, deep GM, and WM. Model fitting was carried out using a variational expectation-maximization (VEM) algorithm [4] which outputs five a posteriori probability maps. They were subsequently converted into three maps corresponding to CSF, GM and WM by combining the ventricular with sulcal CSF maps and the cortical with deep GM maps. The T1 hypointense component in each DGM was computed by summing up GM a posteriori probabilities over each DGM mask and normalizing them by their total volume.
A bilateral two-sample t-test with unequal variances is used for comparing measurements obtained in MS patients and normal subjects, and a p-value < 0.05 is considered as statistically significant.
Results
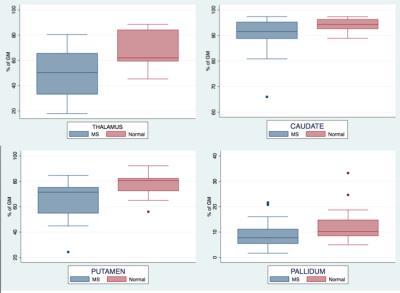
No lesion is observed on the FLAIR images in deep gray matter nuclei of the patients. As shown in Figure 1, the T1 hypointense component is found to be significantly lower in MS patients in the thalamus (48.36 vs 67.63%, p=0.0005), caudate (90 .10 vs 94.07%, p=0.02), putamen (65.81 vs 77.73%, p=0.0043), and pallidum (8.67 vs 12.71%, p=0.048).
Normalized volumes (expressed in % of total intracranial volume) of the patients are significantly reduced in the thalamus (0.909 vs 0.995, p=0.006) and the total brain white matter (28.24 vs 30.45, p=0.013). The observed reductions do not reach significance in the caudate (0.625 vs 0.654, p=0.3), the putamen (0.926 vs 0.978, p=0.11), and the pallidum (0,257 vs 0.276, p=0.088).
Discussion
Our results exhibit significant thalamic volume reduction in MS patients which was previously reported by neuropathological studies [2,5]. More importantly, our data show that the T1 hypointense component of the thalamus and putamen is significantly reduced in relapsing-remitting MS patients. This finding corroborates the observations of Bonnier et al. [3] that longer T1 values in thalamus and putamen will result in more hypointense MR signals in such nuclei for MS patients. This is consistently confirmed by our algorithm. Our automated deep gray matter component evaluation could therefore be considered as a possible marker of neuronal damage enabling image-guided diagnosis based on a conventional MPRAGE scan. To confirm this hypothesis, a longitudinal follow-up study should be conducted as a next step.Acknowledgements
No acknowledgement found.References
1. Stadelmann C, Wegner C, Brück W. Inflammation, demyelination and degeneration – recent insights from MS pathology. BBA – Molecular Basis of Disease 2011;1812(2):275-282
2. Vercellino M, Masera S, Lorenzatti M, Condello C, Merola A, Mattioda A, Tribolo A, Capello E, Mancardi GL, Mutani R, Giordana MT, Cavalla P. Demyelination, inflammation and neurodegeneration in MS deep gray matter. Journal of Neuropathology and experimental neurology 2009;68(5):489-502
3. Bonnier G, Kober T, Schluep M, Du Pasquier R, Krueger G, Meuli R, et al. (2016) A New Approach for Deep Gray Matter Analysis Using Partial-Volume Estimation. PLoS ONE 11(2):e0148631. doi:10.1371/journal.pone.0148631
4. Schmitter D, Roche A, Maréchal B et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. Neuroimage Clinical 2015; 77-17
5. Cifelli A, Arridge M, Jezzard P, Esiri M, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Annals of neurology 2002;52(5):650-653