2526
Correlation between thalamic volume and cognitive impairment in patients with MS using a high-efficiency semi-manual segmentation approach1Hoglund Brain Imaging Center, University of Kansas Medical Center, Kansas City, KS, United States, 2Psychology, University of Kansas, 3Neurology, University of Kansas Medical Center, Kansas City, KS, United States, 4Molecular & Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, United States
Synopsis
Thalamic pathology has been linked to long-term accumulation of disability and cognitive impairment in MS. However, assessment of thalamic volume is highly challenging for automatic as well as manual segmentation techniques. The use of multiple image contrasts may improve segmentation quality. We investigated correlations of thalamic volume and cognitive performance in MS. We evaluated automatic segmentation and our new semi-manual segmentation using T1 and proton-density MRI. Results based on FreeSurfer segmentation failed to yield correlations of thalamic volume with cognitive performance in MS patients. Using semi-manual segmentation, significant correlations were found between cognitive impairment and regional thalamic atrophy in MS.
Introduction
Pathology of the thalamus may be an early indicator of long-term accumulation of disability and cognitive impairment in multiple sclerosis (MS) [1-3]. Assessment of thalamic volume requires anatomical image segmentation, which is often performed using automated tools such as FreeSurfer [4] and FSL:FIRST [5]. However, accurate segmentation of the thalamus is highly challenging due to a lack of contrast and vague boundaries of the surrounding anatomy. Segmentation errors tend to reduce the apparent correlation with other clinical measures, resulting in a failure to yield significance in correlations with cognitive performance. Recent developments in the use of multiple image contrasts for both automatic and manual thalamus segmentation [6-7] underscore a need to improve segmentation methods, particularly deep brain structures including the thalamus. In this study, we aimed to investigate 1) the reliabiblity of automatic segmentation and our new semi-manual segmentation using multiple image constrasts and 2) correlations of thalamic volume and cognitive performance (e.g., information processing speed and memory) in MS.Methods
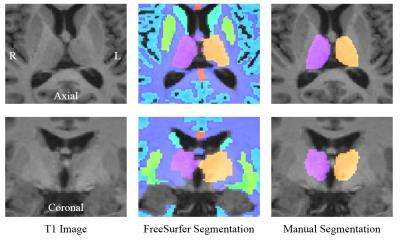
A total of 66 patients with MS (MS) and 31 healthy controls (HC) were studied. The ratio of females to males was MS 37/29 and HC 15/16. There were no significant differences between groups in terms of age, education or sex. Semi-manual segmentation of the thalamus was performed based on co-registered MPRAGE and proton density-weighted images [Jim6, Xinapse Systems]. Our new semi-manual segmentation workflow uses a novel interpolation scheme, which produces smoothly contoured 3D segmentation from a reduced number of manually edited slices as reported previously [8]. Automatic segmentation of the thalamus was performed using FreeSurfer based on the MPRAGE images. The left, right and combined thalamic volumes were measured from both FreeSurfer and semi-manual segmentation methods. All volumes were normalized by the volume of the intracranial cavity (determined by manual segmentation). Subjects performed cognitive tests including the Symbol Digit Modalities Test (SDMT), Rey Auditory Verbal Learning Test (RAVLT) and Brief Visual Memory Test (BVMT). All MRI data were acquired using a 3 T Siemens scanner (Skyra, Siemens, Erlangen, Germany). MRI included T2 and proton density-weighted MRI using a dual-echo spin-echo sequence (TE = 9, 90 ms, TR = 4000 ms, slice thickness = 3 mm, FOV = 240 x 240 mm2, matrix = 256 x 256, and scan time = 7 min) and 3D T1-weighted MRI using an MPRAGE sequence (TE/TR = 4.4/2500 ms, TI = 1100 ms, resolution = 1 x 1 x 1 mm3, and scan time = 8 min).Results and Discussion
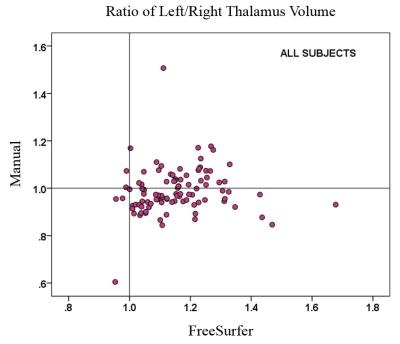
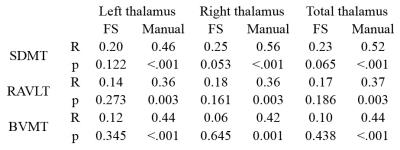
The right and total thalamic volumes were significantly lower in patients with MS for both FreeSurfer and semi-manual segmentation. FreeSurfer segmentation overestimated the left thalamic volume compared with semi-manual segmentation, and the overestimation was more pronounced in MS than in HC. The difference between MS and HC in left thalamic volume was not significant with either FreeSurfer or semi-manual segmentation. However, the correlation between left and right thalamic volumes was smaller for FreeSurfer (0.77) than for semi-manual segmentation (0.92). The correlations between FreeSurfer and semi-manual volumes were 0.50 (left), 0.66 (right), and 0.62 (total). The correlations of thalamic volumes with cognitive performance of patients with MS were significant only with semi-manual segmentation of the thalamus (Table 1). Significant correlations were found between cognitive impairment and regional thalamic atrophy in MS only for the volumes obtained by semi-manual segmentation. FreeSurfer tended to overestimate the left thalamus volume and yielded overall poor correlation with cognitive impairment in MS based on left, right and combined hemispheres. Our findings based on semi-manual segmentation support a link between thalamic pathology and impairment of memory and information processing speed. However, a failure to find correlations with the FreeSurfer results suggests a need for caution in using automated segmentation tools. We conclude that semi-manual segmentation of the thalamus from multiple MRI contrasts demonstrated higher accuracy, while the use of an improved interpolation technique greatly facilitated the manual editing task. This method may allow larger studies to benefit from reliable anatomical volume measures with improved segmentation.Acknowledgements
This study was partly supported by National Multiple Sclerosis Society (RG 4495-A-4) and NIH (UL1TR000001, P20GM103418). The Hoglund Brain Imaging Center is supported by the NIH (S10RR029577) and the Hoglund Family Foundation.References
Mesaros, S. et al. Am J Neuroradiol; 32(6), 1016-1020 (2011).
Minagar, A. et al. Neurology; 80(2), 210-219 (2013).
Schoonheim, M. M. et al. Neurology; 84(8), 776-783 (2015).
Fischl, B. et al. Neuron; 33(3), 341-355 (2002).
Patenaude, B. et al. Neuroimage; 56(3), 907-922 (2011).
Spinks, R. et al. Neuroimage; 17(2), 631-642 (2002).
Power, B.D. et al. Psychiatry Res.; 232(1), 98-105 (2015).
Adany, P. et al. Proc. Intl. Soc. Mag. Reson. Med; 23, 3755 (2015).
Figures