2524
Spinal Cord MRI Water Diffusion Alterations are Linked to Early Axonal Degeneration in the YFP, G93A-SOD1 mice.1Anatomy and Cell Biology, University of Illinois at Chicago, Chicago, IL, United States, 2Biomedical Engineering, University of Illinois at Chicago, Chicago, IL, United States, 3Biochemistry and Molecular Biology, University of Florida, Gainesville, FL, United States
Synopsis
Amyotrophic lateral Sclerosis (ALS) is characterized by progressive degeneration of spinal cord motor neurons. To address the role of axonal pathology in ALS, we generated a YFP,G93A-SOD1 reporter mice. Our goal in this study is to evaluate if presymptomatic alterations in MRI water diffusion in the YFP,G93A-SOD1 mice are related to alterations in axonal connectivity by histological methods. Results showed presymptomatic changes in diffusion parameters are associated to specific structural changes in axonal population. The use of this new animal model will help us to understand the structural basis of changes in water diffusion in ALS.
Target audience:
Neuro-radiologists in the area of neurodegenerative disorders, principally on Amyotrophic Lateral Sclerosis.Purpose:
Amyotrophic lateral Sclerosis (ALS) is characterized by progressive degeneration and death of motor neurons. Among several mouse models available, G93A-SOD1 represent the best-characterized one. The significant parallel between G93A-SOD1 mice phenotype and human ALS made this model a standard for pre-clinical screening of ALS therapies. The early motor effects observed in lower extremities of this animal model, which are similar to the initial clinical presentation in patients with ALS, focused our studies in the white matter (WM) spinal cord (SC). Furthermore, extensive pathological evidence suggests that axonal degeneration is an early and critical pathogenic event in ALS. To address the role of such axonal pathology, we generated a transgenic animal crossing the G93A-SOD1 with a yellow fluorescent protein (YFP) reporter mouse. Thus, the aim of this study is to determine if early MRI water diffusion changes in the YFP, G93A-SOD1 mice are associated to alterations in axonal structure and connectivity using comprehensive histological methods.Methods:
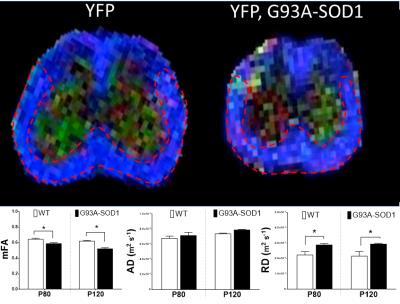
Animal tissues were obtained at 80 and 120 days of age (P80 and P120 groups) in accordance with institutional Animal Care and use committee regulations. MRI studies: Paraformaldehyde-fixed spinal cords (SCs) from YFP (n=4) and YFP,G93A-SOD1 (n=4) from presymptomatic (P80) and post symptomatic (P120) mice were placed in individual 5 mm NMR tubes (New Era, NJ) and immersed in Fluorinert oil. MRI scanning were achieved using a 17.6 T, 31 cm bore Agilent MRI scanner (Santa Clara, CA) with a 25 mm Quad Transceiver mouse coil. Careful manual shimming was performed before diffusion measurement. Such measurements were obtained by using a spin-echo diffusion weighted sequence with the following acquisition parameters: TR = 4000 ms, TE = 28 ms, NEX = 2, FOV = 20 × 20 x9 mm, matrix size = 133 ×133 x 60, image resolution 150x150 μm and b = 700, 2500 s/mm2). We used 12 and 64 directions of diffusion gradients, DWI acquisition time was 6 hours. for b=700, and 19 hours. for b=2500. Histological Analysis: SCs were processed for confocal fluorescence microscopy following standard procedures previously described 1. Antibodies against Myelin Basic Protein (MBP) were used to assess myelin integrity. Data Analysis: Image post-processing was performed offline using DTI Studio (Freeware, Johns Hopkins University). For mean fractal anisotropy (mFA), axial diffusivity (AD) and radial diffusivity (RD) analysis, region of interests (ROIs) were manually selected and centered within the anterolateral funiculi (ALF) (Fig.1).Results:
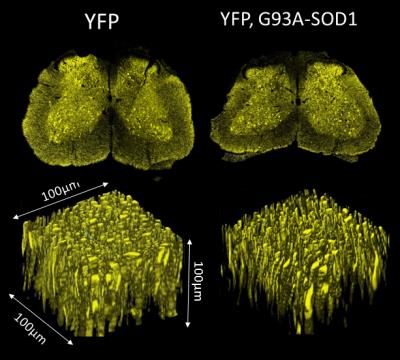
A decrease in mFA values was observed in the WM ALF of the YFP,G93A-SOD1 mice at P80 and P120. Increases in RD were also observed in the same SC regions. Fluorescence microscopy in similar ROIs demonstrated a significant reduction in both axonal area (AA) and axonal density (AD) for G93A-SOD1 mice compared to WT controls (P80) (n=3). (Fig2). Tractography-based data obtained from mFA maps further revealed a similar reduction in the mean length of axonal fibers from the YFP, G93A-SOD1 mice.Discussion and Conclusions:
The expression of YFP in neuronal tissue, showed axonal fiber morphology and integrity in great detail. Thus, confocal microscopic data corroborated the structural changes associated with the alterations of water diffusion parameters. Our studies are consistent with presymptomatic axonal degeneration being detectable by specific MRI techniques. The evaluation of presymptomatic structural changes using YFP,G93A-SOD1 reporter mice provides a cellular basis for the water diffusion alterations detected by MRI imaging. The use of this animal model could help us to find more accurate diffusion models 2, to evaluate early structural biomarkers to expand therapeutic windows and to assess the efficacy of medical strategies aimed at preserving axonal connectivity in ALS.Acknowledgements
The authors wish to acknowledge the support from the Chicago Biomedical Consortium grant (R. Gatto).
References
1 - Gatto RG et al. Analysis of YFP (J16)-R6/2 reporter mice and postmortem brains reveals early pathology and increased vulnerability of callosal axons in Huntington's disease. Hum. Mol. Genet. 2015 Sep 15; 24(18):5285-98.
2 - Magin RL et al. Characterization of Anomalous Diffusion in Porous Biological Tissues Using Fractional Order Derivatives and Entropy Microporous Mesoporous Mater. 2013 Sep 15; 178: 39–43.
Figures