Mami Iima1,2, Akira Yamamoto1, Ichiro Tateya3, Morimasa Kitamura3, Atsushi Suehiro3, Yo Kishimoto3, and Kaori Togashi1
1Department of Diagnostic Imaging and Nuclear Medicine, Graduate Schoolof Medicine, Kyoto University, Kyoto, Japan, 2Hakubi Center for Advaned Research, Kyoto University, Kyoto, Japan, 3Department of Otolaryngology, Head and Neck Surgery., Graduate School of Medicine, Kyoto University, Kyoto, Japan
Synopsis
The
association of diffusion parameters in patients with head and neck cancers was
investigated using the different diffusion times. Although ADCo significantly
decreased (p<0.05) and fIVIM increased (p<0.05) using 51ms compared to 19.1ms,
there was no difference of K values. The effects of the diffusion time on IVIM
and non-Gaussian diffusion parameters are not clear in head and neck cancers. Our
preliminary study requires further validation with shorter diffusion time or
better SNR.
Introduction
Diffusion
MRI is found to be useful for the tumor characterization without the need for
the contrast agents (1). Recently some groups have reported the possibility of different compartments of tissue molecules in
the brain (2) or brain tumor xenograft models (3,4) using different diffusion
times. Diffusion hindrance is supposed to increase with longer diffusion time,
as more water molecules hit obstacles, such as cell membranes, the density of
which increases in cancer tissues. Thus, our purpose was to investigate the
association of diffusion parameters in patients with head and neck cancers, using
the different diffusion times.Material and Methods
This IRB
approved prospective study included 10 patients diagnosed
as head and neck tumors. Head and
neck MRI was performed using a 3-T system (Prisma and Skyra; Siemens Healthcare) equipped with a
dedicated head and neck coil. DWI MRI (WIP) images were acquired using 2 different diffusion times
(diffusion gradient duration(δ): 12ms, and diffusion
gradient separation(Δ): 23.1ms and 55ms, resulting
in the effective diffusion time: 19.1 and 51ms), 8 b values of 0, 100, 200,
600, 1000, 1800, 2600, 3400 sec/mm2; repetition time/echo time; 4,900/87 ms; FOV: 180×180 mm2; matrix: 200×200; slice thickness: 4.0 mm;
bandwidth 1680 Hz/Px, echo spacing: 0.71 ms and the total acquisition time was
5 min 14 sec.
ROIs were placed onto the lesion and images
processing was performed using software implemented in Matlab (Mathwork, Natick,
MA) comprising the following steps:
1/The corrected diffusion signal acquired
with b>200 s/mm² was fitted using the kurtosis diffusion model to estimate
ADCo and K (5):
S/So = exp[-bADCo + K(bADCo)²/6] [1]
where So is the theoretical signal acquired at b=0, ADCo the virtual ADC which would be obtained when b approaches 0, K the kurtosis parameter.
2/Then, the
fitted diffusion signal component was subtracted from the corrected raw signal
acquired with b<200s/mm² and the remaining signal was fitted using the IVIM
model (5) to get estimates of the flowing blood fraction, fIVIM, and
the pseudodiffusion, D*.
3/Synthetic
ADC encompassing both Gaussian and non-Gaussian diffusion effects (6), sADCLb-Hb,
was defined using only 2 b values as:
sADC Lb-Hb, = ln [S(Lb)/S(Hb)]/(Hb-Lb) [2]
here Lb is a low “key” b value and Hb is
high “key” b value seleced to provide the highest sensitivity to
non-Gaussian diffusion Lb and Hb: 200 and 1800
has been used in this study.
Results
2 maxillary cancer, 6 pharyngeal cancer, and 2 laryngeal cancer were included
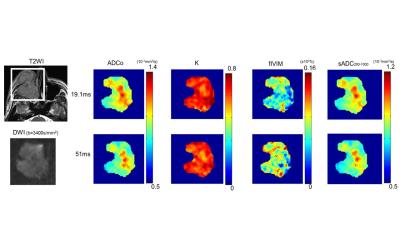
in the study. The representative DWI/IVIM parametric maps of maxillary cancer are
shown in Figure 1. ADCo value has decreased with the increase of diffusion time. There was an increase of fIVIM value with the diffusion time increased. There was no significant difference of K or sADC 200_1800 between the different diffusion times. Figure 2 demonstrates the comparison
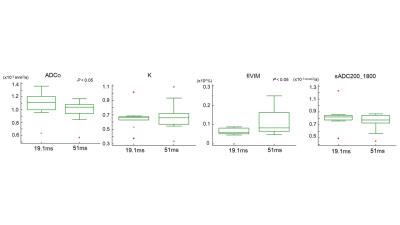
of DWI/IVIM parameters depending on the different diffusion times (19.1ms VS.
51ms). There was a decrease of ADCo (p<0.05) as well as increase of fIVIM (p<0.05) values using 51ms compared to 19.1ms.There was no significant difference of K between different diffusion times. sADC200_1800 value slightly decreased using 51ms, with no significant difference (p=0.13).
Discussion
The
decrease of ADCo values with the increase of diffusion time are well in
agreement with the literature (4,7). However, no significant difference was
observed in K value, which was not in agreement with the study using HCC
xenograft model in mice (7). Considering no change of K value with different
diffusion times, ADCo decrease and fIVIM increase might result from the
artifact from the fitting rather than the increase of restricted diffusion in
tumors (increased hindrance such as cell membranes). Sufficient SNR is important to evaluate the DWI images, particulary at high b values in head and neck region. The clinical investigation
to associate IVIM/DWI parameters in tumors with different diffusion times has
not been performed elsewhere as far as we know, and the development of clinical
MRI scanner allowing shorter diffusion times such as OGSE and better SNR would
be expected to investigate these effects further. Conclusion
Although ADCo significantly decreased and fIVIM increased using 51ms
compared to 19.1ms, the effects of the diffusion time on IVIM and non-Gaussian
diffusion parameters are not clear in head and neck cancers. Our preliminary
study requires further validation with shorter diffusion time or better SNR.Acknowledgements
The authors would like to thank Mr. Yuta Urushibata and Mr.Katsutoshi Murata from Siemens Healthcare K.K. for their technical support in this work, and Dr. Thorsten
Feiweier from Siemens Healthcare for the support
in providing WIP sequence.
References
(1) Le Bihan D et al. Diffusion Magnetic Resonance Imaging: What Water Tells Us about
Biological Tissues. PLoS Biol. 2015 Jul; 13(7): e1002203
(2) Pyatigorskaya
N et al. Relationship between the diffusion time and the diffusion MRI signal
observed at 17.2 Tesla in the healthy rat brain cortex. Magn Reson Med.
2014;72:492-500
(3) Reynaud
O et al. Surface-to-volume ratio mapping
of tumor microstructure using oscillating gradient diffusion weighted imaging.
Magn Reson Med. 2016 Jul;76:237-47
(4) Hope
et al. Demonstration of Non-Gaussian Restricted Diffusion in Tumor Cells Using
Diffusion Time-Dependent Diffusion-Weighted Magnetic Resonance Imaging
Contrast. Front Oncol. 2016; 6:179
(5) Iima
M et al. Quantitative Non-Gaussian Diffusion and Intravoxel Incoherent Motion
Magnetic Resonance Imaging: Differentiation of Malignant and Benign Breast
Lesions. Investigative Radiology 2015:50:205-11
(6) Iima
M et al. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past,
Present and Future. Radiology 2016;278:1
(7) Iima
M et al. Investigation of diffusion signal behavior at different diffusion
times in a human hepatocellular carcinoma xenograft model. Proceedings of the
24th Annual Meeting of ISMRM, Singapore, 2016, p. 3418