2518
Atherosclerotic carotid plaque composition using in-vivo 3T, ex-vivo 7T MRI and histology1Clinical Physics and Bioengineering, University of Glasgow, Glasgow, United Kingdom, 2NHS Greater Glasgow and Clyde, Glasgow, United Kingdom, 3GEMRIC, University of Glasgow, Glasgow, United Kingdom, 4Neuropathology, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom, 5Vascular Surgery, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom, 6Center for Stroke and Brain Imaging, University of Glasgow, Glasgow, United Kingdom, 7Institute of Neurological Siences, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom
Synopsis
Atherosclerotic carotid plaque morphology and plaque composition may identify unstable or vulnerable plaque that defines higher risk. The aim of this study is to evaluate the ability to identify all major carotid plaque components in in-vivo 3T, ex-vivo 7T MRI and correlation with histology.
Introduction
Surgical excision of atherosclerotic carotid plaque (carotid endarterectomy, CEA), based on the extent of luminal narrowing, reduces risk of subsequent stroke. However, 70% of patients with severe stenosis remain stroke-free over the next 5 years with medical therapy alone1. Outcomes from CEA could be improved by targeting treatment at high-risk subgroups. Atherosclerotic plaque morphology and plaque composition may identify unstable or vulnerable plaque that defines higher risk. Recent publications have shown that in-vivo 1.5T MRI can identify the main components of the atherosclerotic plaque, such as the lipid–rich/necrotic core (LR/NC), calcification and haemorrhage. This study aims to evaluate the ability to identify all major carotid plaque components in in-vivo 3T, exvivo 7T MRI and correlation with histology.Methods
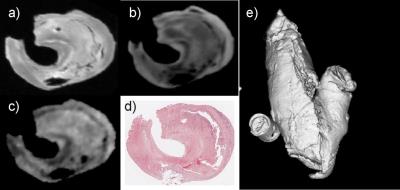
Datasets were obtained from 30 selected symptomatic stroke patients (71±15 years). Subjects were imaged using 3T MR scanner (Sigma Excite, GE). T1-weighted (T1-w) pre/post contrast, T2-weighted (T2-w), Proton Density-weighted (PD-w) and MRA time-of-flight (ToF) scans were carried out. T1-w, T2-w and PD-w were carried out with 0.27x0.27x2.8 mm3 resolution and ToF 0.46x0.46x1.9 mm3. From the patients studied in-vivo, 14 of them underwent CEA. Thirty-three endarterectomy cross-sections, from 13 patients, were studied. The specimens from these patients were imaged on a Bruker Biospec Avance system using a 7T horizontal 30 cm bore magnet. Carotid plaque specimens were imaged in a sealed syringe filled with fomblin, to reduce susceptibility artefacts. A small phantom containing MgCl2 was placed within the field of view as a standard. T1-w, T2-w (100x100x100 μm3 isotropic resolution) and diffusion weighted images (DWI) (181x181x181 μm3 isotropic resolution) were carried out. Serial sections of the specimens were taken and stained with haematoxylin-eosin and Elastic van Gieson. Digital images of the histological preparations were acquired at 0.54x0.54 μm2 resolution. Histological correlation with the in-vivo 3T and ex-vivo 7T MRI data was carried out. All the images of the different modalities were co-registered using a commercial package Analyze (Biomedical Imaging Resource, Mayo Foundation).
Semiautomated segmentation methods were used to measure areas of different plaque components2. Bland-Altman plots and mean difference with 95% confidence interval were carried out.
Results
There was general quantitative agreement between areas derived from semi-automated segmentation of MRI data and histology measurements.
3T MRI vs Histology
The mean differences and 95% confidence bounds in the relative to total plaque area between 3T versus Histology were: fibrous tissue 4.99%(-4.56 to 14.56), lipid-rich/necrotic core (LR/NC) with hemorrhage -1.81%(-14.11 to 10.48), LR/NC without hemorrhage -2.43%(-13.04 to 8.17), and calcification -3.18%(-11.55 to 5.18).
7T MRI vs Histology
The mean differences and 95% confidence bounds in the relative to total plaque area between 7T and histology were: fibrous tissue 3.17%(-3.17 to 9.52), LR/NC with hemorrhage -0.55%(-9.06 to 7.95), LR/NC without hemorrhage -12.62%(-19.8 to -5.45), and calcification -2.43%(-9.97 to 4.73).
Conclusions
This study provides evidence that semi-automated segmentation of 3T/7T MRI techniques can help to determine atherosclerotic plaque composition. The high resolution of ex vivo 7T data was able to highlight greater detail in the atherosclerotic plaque composition.Acknowledgements
No acknowledgement found.References
1. Hankey GJ. Stroke Treatment and Prevention: An Evidence-Based Approach. New York: Cambridge University Press. 2005:255-87.
2. Saam T et al Arteriosclerosis, Thrombosis, and Vascular Biology 2005; 25:234-239.