2509
Toward Clinical Translation of Quantitative Spinal Cord MRI: Serial Monitoring to Identify Disease Progression in Patients with Degenerative Cervical Myelopathy1Neurosurgery, University of Toronto, Toronto, ON, Canada, 2Electrical Engineering, École Polytechnique de Montréal, 3Neurosurgery, University of Calgary, Calgary, AB, Canada, 4Medicine, University College Cork, Cork, Ireland, 5Medical Imaging, University of Toronto, Toronto, ON, Canada
Synopsis
Degenerative cervical myelopathy (DCM) is a common cause of disability, but mild patients are often managed non-operatively and monitored for deterioration. Popular clinical assessment tools are insensitive to detect subtle disease progression. In this study, we employ multi-parametric spinal cord MRI to monitor 15 DCM patients for progression over a 1-year period, in addition to a comprehensive battery of clinical assessments. The MRI results detected progressive tissue injury in 6/7 patients with definite clinical progression and 5 additional patients (4 of which had borderline clinical progression). These MRI assessments are now being incorporated into clinical practice to inform surgical decision-making.
INTRODUCTION
Degenerative cervical myelopathy (DCM) is a common condition in which the discs, ligaments, and bones cause extrinsic compression of the spinal cord (SC). Cervical SC compression is often asymptomatic,1 but when symptoms arise they can cause severe impairments (loss of hand dexterity, gait imbalance, numbness, weakness, and urinary incontinence).2 Decompressive surgery is used to treat patients with advanced disease, but many patients with mild symptoms are treated non-operatively. The natural history of DCM is variable, ranging from long-term stability to rapid decline.2 Clinical progression is often subtle and can be difficult to confidently determine by clinicians, while electrophysiology is of little use.3 MRI techniques that can provide direct measures of tissue injury, such as axonal injury, demyelination, and atrophy, could have a major impact. This longitudinal prospective study assesses the utility of serial multi-parametric SC MRI at 1-year follow-up to detect disease progression in DCM patients managed non-operatively, in comparison with a battery of clinical assessments.METHODS
Fifteen DCM patients with baseline and 1-year follow-up data were included. Clinical data included mJOA, QuickDASH, hand dexterity (GRASSP-CM), grip strength, hand sensation (monofilaments), Berg Balance, and gait stability ratio (GaitRITE). MRI data (3T GE) included T2-weighted (T2w) imaging (FIESTA-C, 0.8x0.8x0.8mm3), diffusion tensor imaging (DTI, ssEPI, 1.25x1.25x5mm3), magnetization transfer (MT; SPGR with/without prepulse, 1x1x5mm3), and T2*-weighted (T2*w) imaging (MERGE, 3 echoes, 0.6x0.6x4mm3) covering C1-C7 (Figure 1).4
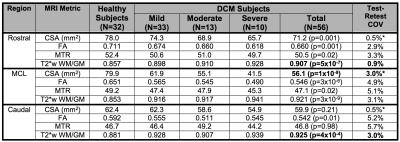
MRI data were analyzed with the Spinal Cord Toolbox (SCT)5 to calculate SC cross sectional area (CSA), fractional anisotropy (FA), MT ratio (MTR), and T2*w white to grey matter signal intensity ratio (T2*w WM/GM). Regions of interest (ROIs) were SC for CSA and WM for other metrics, extracted from maximally compressed (MCL), rostral (C1-C3), and caudal (C6-C7) levels. Ten of these metrics were employed, having previously demonstrated significant differences between DCM and healthy subjects (Table 1).6
Disease progression was arbitrarily defined as ≥ 3 clinical measures worsening by 5%, and borderline progression was noted when 1-2 clinical measures worsened. Changes in MRI metrics were converted to z scores, using previously established test-retest sample standard deviation as the denominator (Table 1).4,7 Individual z scores were averaged to yield a Composite Score (t distribution, 10 degrees of freedom) with standard error estimated as 1/ √10). Overall α was set to 0.05 and MRI progression was defined as any z score < -2.65 or Composite Score t10 < - 3.30 (p<0.004, single-tailed, corrected for multiple comparisons).
RESULTS
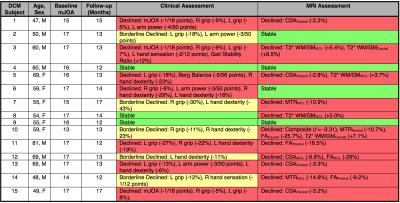
Seven of 15 patients showed definite clinical worsening, 5 showed borderline progression, and 3 remained stable (Table 2). Clinical changes were seen using grip strength (8/12), hand dexterity (7/12), arm power (4/12), mJOA (3/12), hand sensation (2/12), gait stability ratio (1/12), and Berg Balance (1/12).
Eleven of 15 patients showed MRI progression, with 5 showing changes in ≥ 2 metrics and 6 in a single metric. Among patients with definite clinical worsening, 6/7 showed MRI progression; 4/5 patients with borderline clinical progression showed MRI progression; 1/3 clinically stable patients showed progression on MRI. MRI progression was seen using CSARostral (4/11), T2*w WM/GMMCL (3/11), T2*w WM/GMCaudal (2/11), FARostral (2/11), MTRMCL (2/11), Composite Score (1/11), CSAMCL (1/11), FAMCL (1/11), FACaudal (1/11), and MTRRostral (1/11).
DISCUSSION
Although individual MRI measures showed limited responsiveness to disease progression, the multi-parametric approach appeared to provide an accurate assessment of tissue injury progression. Interestingly, MRI showed progression more often than clinical measures (excluding borderline cases), possibly because incremental injury does not necessarily manifest with neurological/functional deficits, due to neuroplasticity, behavioural adaption, and the subjective or transient nature of symptoms. It is difficult to determine the true accuracy of clinical and MRI measures to quantify injury, as the ground truth in living subjects is unknown. The most commonly used clinical tool is mJOA, but this was unresponsive to progression in our data. Our battery of clinical assessments appear to be more sensitive and responsive to subtle decline in functional status; however, the clinical significance of these observations requires validation in a larger cohort. Overall, the MRI assessment confirmed the presence of subtle disease progression in most cases, and was performed using clinically feasible methods that could be implemented on any 3T scanner.
Our initial results, in a small prospective cohort of patients using a novel MR imaging protocol in conjunction with sensitive clinical assessments, appear to add valuable information for surgical decision-making in mild DCM. We are now disclosing the results of these MRI assessments to patients and their spine surgeons (while highlighting their preliminary nature), constituting an important step toward clinical translation of these techniques.
Acknowledgements
This research received funding support from Rick Hansen Institute, as part of the Riluzole in Spinal Cord Injury Study (RISCIS), which is also supported by AOSpine North America, AOSpine International SCI Knowledge Forum, and the North American Clinial Trials Network (NACTN) of the Christopher and Dana Reeve Foundation. This research also received support from the Dezwirek Foundation, the Sherman Clinical Research Unit, and the Gerald and Tootsie Halbert Chair in Spinal Cord Research. Dr. Martin received post-doctoral fellowship support from Canadian Institutes of Health Research.References
1. Wilson JR, Barry S, Fischer DJ, et al. Frequency, Timing, and Predictors of Neurological Dysfunction in the Nonmyelopathic Patient With Cervical Spinal Cord Compression, Canal Stenosis, and/or Ossification of the Posterior Longitudinal Ligament. Spine. 2013;38(22):S37-S54.
2. Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409-21.
3. Kerkovsky M, Bednarik J, Dusek L, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976). 2012;37(1):48-56.
4. Martin AR, De Leener B, Cohen-Adad J, Aleksanderek I, Cadotte DW, Fehlings MG. A Clinically Feasible Quantitative MRI Protocol to Assess Tissue Injury in the Cervical Spinal Cord Using DTI, MT, and T2*-Weighted Imaging: Reliability and Variations with Confounding Variables. The Spine Journal Oct 2016;16(10) Suppl S199–S200.
5. De Leener B, Lévy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. NeuroImage Epub: Oct 5, 2016.
6. Martin AR, De Leener B, Cohen-Adad J, et al. Microstructural MRI Quantifies Tract-Specific Injury and Correlates With Global Disability and Focal Neurological Deficits in Degenerative Cervical Myelopathy. Neurosurgery. 2016 Aug;63 Suppl 1:165.
7. Kearney H, Yiannakas MC, Abdel-Aziz K, et al. Improved MRI quantification of spinal cord atrophy in multiple sclerosis. J Magn Reson Imaging. 2014;39(3):617-623.
Figures