2504
Benefits of high-resolution QSM acquisition protocol for DBS surgery planning1Meing School of Biomedical Engineering, Cornell University, Ithaca, NY, United States, 2Radiology, Weill Cornell Medical College, New York, NY, United States, 3Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan, People's Republic of China, 4Weill Cornell Medical College, New York, NY, United States, 5Department of Neurosurgery, Mount Sinai Health System, New York, NY, United States
Synopsis
Deep brain stimulation is a surgical procedure routinely used in the treatment of advanced stages of Parkinson disease. DBS involves implanting of stimulating electrodes inside the patient’s brain, with STN most commonly being the target brain structure. Treatment efficiency and absence of negative side effects is strongly dependent on precision of electrode placement; therefore, high requirements are imposed on preoperative patient imaging for proper identification of anatomy of interest. Histochemical studies suggest that iron (one of the major contrast contributors in QSM) is densely and heterogeneously distributed in STN. Furthermore, it is hypothesized that distribution of iron might be related to functional subdivisions in STN. Thus, DBS surgery planning might benefit from more precise calculation of susceptibility distribution, which would allow observe and characterize gradients in iron concentration in in vivo patient data potentially leading to minimization of non-motor side effects. Accordingly, we develop a high resolution QSM protocol for DBS presurgical MRI protocol.
Purpose
To improve quality of preoperative quantitative susceptibility mapping (QSM) and targeting of subthalamic nucleus (STN) in deep brain stimulation (DBS) using 3T magnetic resonance (MR) imaging.Introduction
Deep brain stimulation is a surgical procedure routinely used in the treatment of advanced stages of Parkinson disease. DBS involves implanting of stimulating electrodes inside the patient’s brain, with STN most commonly being the target brain structure. Treatment efficiency and absence of negative side effects is strongly dependent on precision of electrode placement [1]; therefore, high requirements are imposed on preoperative patient imaging for proper identification of anatomy of interest. Histochemical studies suggest that iron (one of the major contrast contributors in QSM) is densely and heterogeneously distributed in STN. Furthermore, it is hypothesized that distribution of iron might be related to functional subdivisions in STN [2]. Thus, DBS surgery planning might benefit from more precise calculation of susceptibility distribution, which would allow to observe and characterize gradients in iron concentration in in-vivo patient data potentially leading to minimization of side effects. Accordingly, we develop a high resolution QSM acquisition for DBS presurgical MRI protocol.Materials and methods
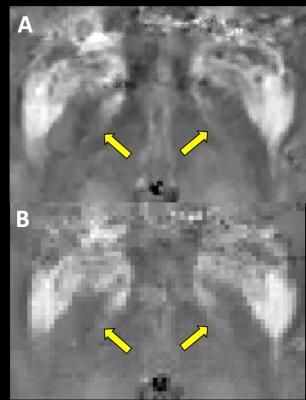
Patients - 13 PD patients referred by neurologists for preoperative MR imaging prior to for DBS surgery (9 men, 4 women, age range 31-81 years, median age 58 years). All acquisitions were performed with a clinical 3T MR system using a standard eight-channel head coil. Protocol - Two multi-echo gradient echo datasets with two different isotropic resolutions were acquired for each volunteers using a spoiled 3D multi-echo GRE sequence. The QSM acquisition was based on the following prescription: FOV 25.6 cm, Phase FOV 0.8, slice thickness 1.0 mm, #TE 11, FA 15, Frequency encodes 320 (256 for low resolution data), Phase encodes 320 (256 for low resolution data), NEX 0.75, BW, 62.5 kHz; Imaging options: ZIP2; phase and frequency encoding directions were further zero-padded to 512 points during DICOM reconstruction. Parallel imaging technique was used with the reduction factor of 2 to accelerate acquisition. QSM was reconstructed for each acquired case using nonlinear MEDI. Anonymized images were presented to three independent graders (two trained neuroradiologists and one neurosurgeon) in randomized order for assessment of STN features. The following scale was employed:
score 0 : STN was not visible;
score 1: the STN-SN complex was poorly visible with fuzzy borders;
score 2: only the superior border of STN and not the border with SN was visible;
score 3: STN was well defined and clearly differentiable from its superior neighbor, presumably ZI, and its inferior neighbor, SN; no gradient is observed in STN area;
score 4: STN was well defined and clearly differentiable from its superior neighbor, presumably ZI, and its inferior neighbor, SN; gradient in STN is readily observed.
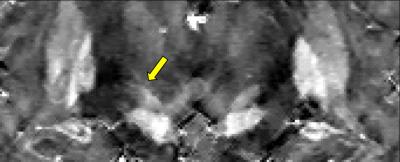
Additionally, one of the graders was asked to perform bilateral segmentation of STNs into hypo- and hyperintense regions on high resolution datasets. Prepared ROIs then were registered to low resolution images, and CNR analysis was carried on both resolution datasets by comparing average QSM values within specified regions versus QSM standard deviation in superior tissue (zona incerta).
Results
Collected scores were evaluated. High resolution datasets were scored higher (average scores 3.4±0.7, 3.7±0.75 and 3.9±0.28) than low resolution images (2.4±0.5, 3.2±0.99 and 3.2±0.55), demonstrating statistically significant change in all evaluations (p<0.05). Percentage of agreement between raters for high resolution cases was also higher (1-2: 62% vs 38%; 1-3: 54% vs 38%; 2-3: 77% vs 38%) which suggested higher rater confidence in identification of structures of interest. Statistical analysis of the CNR measurements for demonstrates statistically significant improvement in visibility of STN hypointense areas in QSM for both left and right sides (average CNR: 6.3±3.7 vs 4.6±2.96 and 6.5±3.6 vs 4.3±3.0, correspondingly) (p<0.05). Meanwhile, no statistically significant change for hyperintense areas was observed.Discussion
Our results indicate that high resolution QSM protocol yielded a superior quality in depiction of STN substructures, which may improve neurosurgical targeting and surgical planning for deep brain stimulator placement used to treat Parkinson disease. sharp structure interface in STN iron concentration has been observed, which means subregions of STN are organized in a continuous manner. The improved CNR and assessment scores suggest that high resolution protocol performs significantly better than typically used clinical protocols.Conclusion
Proposed high resolution protocol might be routinely used in clinical practice for improved outcome of DBS surgery.Acknowledgements
R01NS072370, R01NS090464, R01NS095562References
[1] Guehl et al, Eur J Nurol 2006;13(9):963-971 [2] de Hollander et al, Hum Brain Mapp. 2014 Sep;35(9):4440-9Figures