2502
Lessons for MRI recruitment in movement disorder: Clinical presentation is not related to motion artefacts in arterial spin labelling MRI1CUBRIC, Cardiff University, Cardiff, United Kingdom, 2Neuroscience and Mental Health Research Institute, Cardiff University, 3School of Medicine, Cardiff University, 4School of Physics and Astronomy, Cardiff University
Synopsis
Motion artefacts pose significant problems for the acquisition and analysis of MRI data. In movement disorders, severe motion-related artefacts can result in data being discarded as non-usable. It is not known to what degree clinical movement symptoms can predict in-scanner motion artefacts, and thus, whether researchers can target recruitment for MRI studies based on clinical presentation. Here we investigate whether movement severity in Huntington’s disease, a neurodegenerative movement disorder, can predict in-scanner motion artefacts in arterial spin labelling data. We find that motion magnitude and variability is not more pronounced in Huntington’s disease and not related to symptom severity.
Purpose.
Motion artefacts pose significant problems for the acquisition and analysis of MRI data, especially in EPI-based sequences such as arterial spin labelling (ASL). The risk of severe motion-related artefacts are a concern during recruitment for MRI studies of movement disorders, yet it is not known to what degree clinical presentation affects in-scanner head movement. Recruiting patients who exhibit severe movement inside the scanner results in non-viable data with financial and time costs. Conversely, excluding participants based on their clinical presentation may bias the study towards milder and early disease stages, and in studies of rare diseases, reduce the statistical power.
Here we seek to establish whether clinical parameters in Huntington’s Disease (HD) can predict the magnitude of variability in motion in ASL and can guide recruitment, avoiding the inappropriate inclusion and/or exclusion of patients.
Methods.
Multi-inversion time (TI) PICORE1 pulsed ASL acquisitions were performed on 19 HD patients (45.3 ± 8.8 years old, 13 males) and 18 age and gender matched healthy control participants (3T GE HDx system). Images were acquired with a spiral gradient echo sequence with a variable TR (1100-2000ms) for efficiency (TE = 2.7 ms, 15 slices, slice gap 1 mm, slice delay 52 ms, QUIPSS II2 cutoff TI>700 ms, voxel size 3 × 3 × 8 mm3) and 8 TIs (400,500, 600,700, 1100, 1400, 1700, 2000ms). ASL time series were motion corrected using afni software (afni.nimh.nih.gov; 12 parameter with LPA cost function, 6 parameter warp, and one-pass refining strategy).
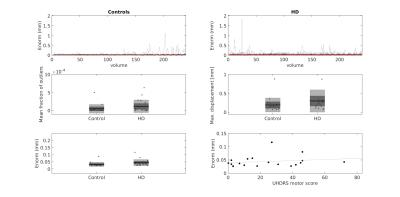
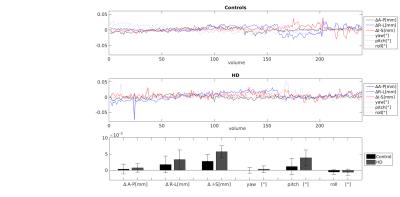
To examine motion magnitude and variability, the Euclidean norm (Enorm; √sum of squares) of the backward distance of the motion parameters was calculated per volume, along with the maximum motion displacement (difference in position) between any volume pair. The number of outliers (>3.5* median absolute deviation distance to the trend) in each volume was examined after 3rd degree legendre polynomial detrending.
Using a linear regression model, the relationship between ASL time series motion and two clinical measures was examined: (1) the Unified Huntington’s Disease Rating Scale (UHDRS) motor score, commonly used to diagnose the clinical onset of HD, with a higher score indicative of more severe movement; (2) the number of CAG repeat lengths, which indicates the genetic load in HD, with a greater CAG repeat length resulting in earlier disease onset with greater symptom severity.
Results.
The mean Euclidean norm across volumes was 36.1% higher in HD participants compared to controls, with the standard deviation 53.9% higher in HD participants; these group differences were not statistically significant (Fig.1, p >0.05). UHDRS motor score and genetic load did not predict the mean Euclidean norm (std. β = 0.38 and -0.15 respectively, both p > 0.05, Fig.1).
In order to examine if the spikes in the motion parameters (Fig.2) were related to HD clinical presentation, the fraction of outliers per volume was calculated. There was no difference between HD and control participants in the mean fraction of outliers across all volumes (control = 0.005 ± 0.012%; HD = 0.012 ± 0.018%, p > 0.05) and the number of outliers was very low (<2 % of voxels affected in both groups). However, a regression model with the genetic disease load (CAG repeat length) and the UHDRS motor score as predictor variables accounted for 37.1% of the variance in mean outlier fraction (p<0.05) with the UHDRS motor score significantly predicting the outlier fraction (std β = 0.80, p < 0.005).
Removing peaks where > 1% of voxels were affected (5 HD volumes, 2 control volumes) had no effect on the results; there was no difference in mean Euclidean norm between HD and control participants (control M=0.032±0.004; HD M=0.043±0.004, p = 0.067), and clinical measures did not predict the Euclidean norm (p>0.05).
Conclusions.
The magnitude of variability in motion in ASL data was not found to be more severe in HD patients compared to controls. Moreover, the clinical motor score in HD patients was not related to the Euclidean norm of motion. However, the clinical severity of motor symptoms did affect the fraction of outliers detected in the data, suggesting that large movements may be problematic in more advanced disease stages.
Together, this data suggests that after screening the data for outliers caused by bulk movements, the clinical presentation of chorea/bradykinesia does not impact data quality (related to head motion) in the MRI environment and should not guide recruitment in HD.
Acknowledgements
We wish to acknowledge the Waterloo Foundation for funding.References
1. Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–249
2. Wong, E. C., Buxton, R. B., & Frank, L. R. (1998). Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magnetic resonance in medicine, 39(5), 702-708.