2501
Measuring exercise-induced cerebrovascular changes in Huntington's Disease using arterial spin labelling (ASL) fMRI1CUBRIC, Cardiff University, Cardiff, United Kingdom, 2Neuroscience and Mental Health Research Institute, Cardiff University, 3Cardiac Services, Cardiff and Vale University Health Board, Cardiff, United Kingdom, 4School of Medicine, Cardiff University, 5Centre for Trials Research, Cardiff University, Cardiff, United Kingdom, 6School of Physics and Astronomy, Cardiff University
Synopsis
Exercise is potentially therapeutic via vascular adaptations (angiogenesis, improved cerebral perfusion and metabolism) however the underlying dynamics are not fully understood. In Huntington´s disease (HD), where the therapeutic potential of exercise is being explored, cerebral vasculature alterations have been reported.
Here we used arterial spin labelling to examine the acute effect of aerobic exercise on the cerebrovasculature in HD patients. We show that genetic disease load is related to both baseline cerebral blood flow (CBF) and the exercise-induced change in CBF.
Purpose
It is increasingly being realised that exercise has
beneficial effects on brain health, and its role as a therapeutic for various
neurological conditions is under investigation. However, the dynamic mechanisms
of adaptive plasticity are poorly understood, with emerging evidence of
exercise-induced cerebrovascular
adaptations (angiogenesis, improved cerebral perfusion and metabolism)1.
In mouse models and patient studies of
Huntington´s disease (HD), cerebral vasculature alterations have been reported2. and
in patients, long-term exercise intervention trials are underway, with the aim
to slow or halt disease progression. Here, using arterial spin labelling
(ASL) we examined whether cerebral blood flow is altered in HD patients at
baseline, and in response to a single session of exercise.
Methods.
18 gene-positive HD patients (11 males, 45.0 ± 8.5 years old) and 18 healthy age-matched controls underwent a baseline MRI scan (3T GE HDx system) with physiological monitoring (heart rate, blood pressure, blood gases). A multi-inversion time PICORE3 pulsed ASL sequence was used to measure cerebral blood flow (CBF, ml/100g/min). Images were acquired with a spiral gradient echo sequence with a variable TR (1100-2000ms) for efficiency (TE = 2.7 ms, 15 slices, slice delay = 52 ms, slice gap = 1 mm, QUIPSS II4 cutoff TI>700 ms, voxel size 3 × 3 × 8 mm3) and 8 inversion times (TIs: 400,500, 600,700,1100, 1400, 1700, 2000ms). A structural FSPGR (1mm3 resolution) was also acquired.
Participants then completed 20-minutes of moderate (60-70% intensity) aerobic exercise on a cycle ergometer. Immediately after, participants underwent a repeat MRI session with the ASL sequence occurring 15 minutes after exercise cessation. Baseline fitness was assessed on a separate day using cardiopulmonary testing.
Perfusion quantification was performed on a voxel-by-voxel basis using a two-compartment model5 with partial volume correction6. Median grey matter CBF values were compared pre- and post-exercise using a repeated-measure ANOVA. The relationship between CAG repeat length, a genetic marker of disease load in HD and CBF was examined.
Results.
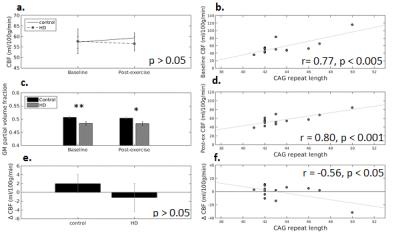
There was no interaction between time (pre- vs. post exercise) and gene status (HD vs. controls) for grey matter CBF, and similarly, no main effect of gene status (Fig, 1a). Cardiorespiratory fitness, assessed on a separate day, was not found to be related to baseline grey matter CBF in HD and control participants alike. When examining the variance within the HD group, CBF was found to be significantly correlated with CAG repeat length (~genetic mutation load) in HD at baseline and post-exercise (Fig. 1b,d, n=13, p < 0.005).
In order to explore individual variability in exercise response, the absolute exercise-induced change in CBF (post-exercise CBF - baseline CBF) was calculated, and found to be negatively related to CAG repeat length in HD participants (Fig. 1f).
The partial volume
fraction of grey matter was significantly lower in HD participants (Fig1c),
however this fraction was not related to CAG repeat length (p>0.05).
Conclusions
We have demonstrated that
grey matter CBF is not altered in HD, in contrast to previous evidence of
cerebrovascular changes in HD postmortem tissue. For both healthy control
participants and HD patients, a single session of aerobic exercise was not
found to significantly affect grey matter CBF. However, both baseline CBF and
the absolute change in CBF following exercise were found to be related to CAG
repeat length, an index of genetic load in HD. This suggests that the
variability in grey matter cerebral perfusion in the HD group is clinically
meaningful, and the relationship with exercise-induced CBF change may suggest
that disease load affects the responsiveness to exercise. Importantly, the
relationship between genetic load and CBF does not appear to be driven by
atrophy-derived partial volume effects.
Acknowledgements
We would like to thank the Waterloo Foundation for funding this research.References
1.Chirico, E.N. et al. 2016. MRI biomarkers of exercise-induced improvement of oxidative stress and inflammation in the brain of old high fat fed ApoE-/- mice. J Physiol. 2016 Sep 19.
2. Drouin-Ouellet J. et al., 2015. Cerebrovascular and blood-brain barrier impairments in Huntington's disease: Potential implications for its pathophysiology.. Ann Neurol. 78(2):160-77.
3. Wong, E.C., Buxton RB, Frank LR. (1997). Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 10:237–249
4. Wong, E. C., Buxton, R. B., & Frank, L. R. (1998). Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magnetic resonance in medicine, 39(5), 702-708.
5. Chappell, M.A., et al. (2010). Separation of macrovascular signal in multi-inversion time arterial spin labelling MRI. MRM. 2010; 63: 1357–1365
6. Chappell MA, MacIntosh BJ, Donahue MJ,Jezzard P, Woolrich MW. (2011). Partial volume correction of multiple inversion time arterial spin labeling MRI data, Magn Reson Med, 65(4):1173-1183.